Two prophylactic medication approaches in addition to a pain control regimen for early medical abortion < 63 days' gestation with mifepristone and misoprostol: study protocol for a randomized, controlled trial
- PMID: 27733165
- PMCID: PMC5062865
- DOI: 10.1186/s12978-016-0246-5
Two prophylactic medication approaches in addition to a pain control regimen for early medical abortion < 63 days' gestation with mifepristone and misoprostol: study protocol for a randomized, controlled trial
Abstract
Background: Pain is often cited as one of the worst features of medical abortion. Further, inadequate pain management may motivate some women to seek unnecessary clinical care. There is a need to identify effective methods for pain control in this setting.
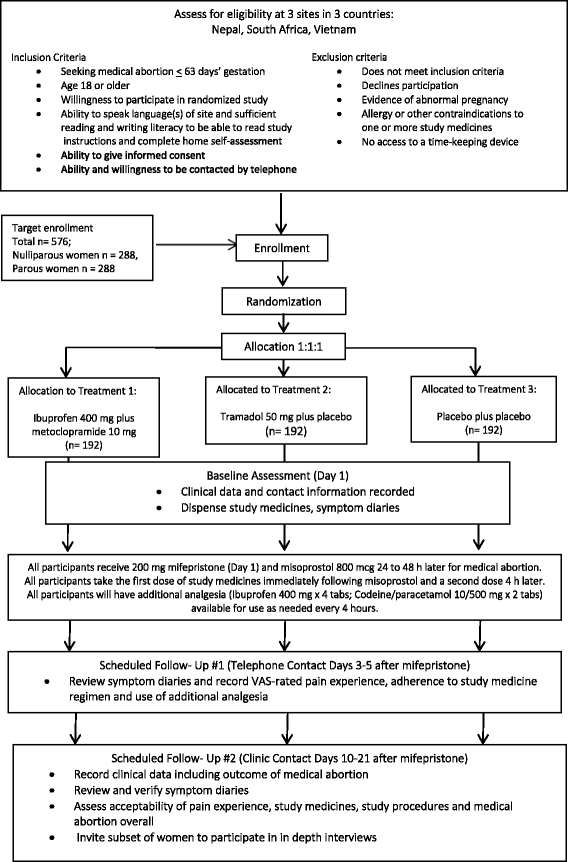
Methods/design: We propose a randomized, placebo-controlled trial. 576 participants (288 nulliparous; 288 parous) from study sites in Nepal, South Africa and Vietnam will be randomly allocated to one of three treatments: (1) ibuprofen 400 mg PO and metoclopramide 10 mg PO; (2) tramadol 50 mg PO and a placebo; or (3) two placebo pills, to be taken immediately before misoprostol and repeated once four hours later. All women will be provided with supplementary analgesia for use as needed during the medical abortion. We hypothesize that women receiving prophylactic analgesia will report lower maximal pain scores in the first 8 h following misoprostol administration compared to women receiving placebos for medical abortion through 63 days' gestation. Our primary objective is to determine whether prophylactic administration of ibuprofen and metoclopramide or tramadol provides superior pain relief compared to analgesia administration after pain begins, measured during the first eight hours after misoprostol administration. Secondary objectives include identifying covariates associated with higher reported pain scores; determining any impact of the study medicines on medical abortion success; and, qualitatively exploring women's physical experiences of medical abortion, especially related to pain, and how can they be improved. Data sources include medical records, participant symptom diaries and interview data obtained on the day of enrollment, during the medical abortion, and at follow-up. Participants will be contacted via telephone on day 3 and return for follow-up will occur approximately 14 days after mifepristone, concluding study participation. A subset of 42 women will also be invited to undergo in-depth qualitative interviews following study completion.
Discussion: Although pain is one of the most common side effects encountered with medical abortion, little is known about optimal pain management for this process. This multi-arm trial design offers an efficient approach to evaluating two prophylactic pain management regimens compared to use of pain medication as needed.
Trial registration: ACTRN12613000017729 (Prospectively registered 8/1/2013).
Keywords: Medical abortion; Mifepristone; Misoprostol; Pain management.
Similar articles
-
Two prophylactic pain management regimens for medical abortion ≤63 days' gestation with mifepristone and misoprostol: A multicenter, randomized, placebo-controlled trial.Contraception. 2021 Mar;103(3):163-170. doi: 10.1016/j.contraception.2020.12.004. Epub 2021 Jan 13. Contraception. 2021. PMID: 33451721 Clinical Trial.
-
Pre-emptive effect of ibuprofen versus placebo on pain relief and success rates of medical abortion: a double-blind, randomized, controlled study.Fertil Steril. 2012 Mar;97(3):612-5. doi: 10.1016/j.fertnstert.2011.12.041. Epub 2012 Jan 20. Fertil Steril. 2012. PMID: 22265034 Clinical Trial.
-
Low-dose mifepristone 200 mg and vaginal misoprostol for abortion.Contraception. 1999 Jan;59(1):1-6. doi: 10.1016/s0010-7824(98)00150-4. Contraception. 1999. PMID: 10342079 Clinical Trial.
-
Mifepristone and vaginal misoprostol on the same day for abortion from 50 to 63 days' gestation.Contraception. 2002 Oct;66(4):225-9. doi: 10.1016/s0010-7824(02)00358-x. Contraception. 2002. PMID: 12413616
-
Early pregnancy loss medical management in clinical practice.Contraception. 2023 Oct;126:110134. doi: 10.1016/j.contraception.2023.110134. Epub 2023 Jul 29. Contraception. 2023. PMID: 37524147 Review.
Cited by
-
Experiences with pain of early medical abortion: qualitative results from Nepal, South Africa, and Vietnam.BMC Womens Health. 2019 Oct 15;19(1):118. doi: 10.1186/s12905-019-0816-0. BMC Womens Health. 2019. PMID: 31615501 Free PMC article.
-
Pain management for medical abortion before 14 weeks' gestation.Cochrane Database Syst Rev. 2022 May 13;5(5):CD013525. doi: 10.1002/14651858.CD013525.pub2. Cochrane Database Syst Rev. 2022. PMID: 35553047 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical