Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial
- PMID: 27733243
- PMCID: PMC5648054
- DOI: 10.1016/S1470-2045(16)30496-X
Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial
Erratum in
-
Correction to Lancet Oncol 2016; 17: 1590-98.Lancet Oncol. 2019 Feb;20(2):e71. doi: 10.1016/S1470-2045(19)30014-2. Lancet Oncol. 2019. PMID: 30712805 No abstract available.
Abstract
Background: Few effective treatments exist for patients with advanced urothelial carcinoma that has progressed after platinum-based chemotherapy. We assessed the activity and safety of nivolumab in patients with locally advanced or metastatic urothelial carcinoma whose disease progressed after previous platinum-based chemotherapy.
Methods: In this phase 1/2, multicentre, open-label study, we enrolled patients (age ≥18 years) with urothelial carcinoma of the renal pelvis, ureter, bladder, or urethra at 16 sites in Finland, Germany, Spain, the UK, and the USA. Patients were not selected by PD-L1 expression, but tumour PD-L1 membrane expression was assessed retrospectively. Patients received nivolumab 3 mg/kg intravenously every 2 weeks until disease progression or treatment discontinuation because of unacceptable toxicity or other protocol-defined reasons, whichever occurred later. The primary endpoint was objective response by investigator assessment. All patients who received at least one dose of the study drug were included in the analyses. We report an interim analysis of this ongoing trial. CheckMate 032 is registered with ClinicalTrials.gov, NCT01928394.
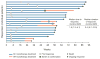
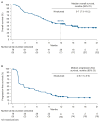
Findings: Between June 5, 2014, and April 24, 2015, 86 patients with metastatic urothelial carcinoma were enrolled in the nivolumab monotherapy group and 78 received at least one dose of treatment. At data cutoff (March 24, 2016), the minimum follow-up was 9 months (median 15·2 months, IQR 12·9-16·8). A confirmed investigator-assessed objective response was achieved in 19 (24·4%, 95% CI 15·3-35·4) of 78 patients. Grade 3-4 treatment-related adverse events occurred in 17 (22%) of 78 patients; the most common were elevated lipase (four [5%]), elevated amylase (three [4%]), and fatigue, maculopapular rash, dyspnoea, decreased lymphocyte count, and decreased neutrophil count (two [3%] each). Serious adverse events were reported in 36 (46%) of 78 patients and eight (10%) had a serious adverse event judged to be treatment related. Two (3%) of 78 patients discontinued because of treatment-related adverse events (grade 4 pneumonitis and grade 4 thrombocytopenia) and subsequently died.
Interpretation: Nivolumab monotherapy was associated with a substantial and durable clinical response and a manageable safety profile in previously treated patients with locally advanced or metastatic urothelial carcinoma. These data support further investigation of nivolumab monotherapy in advanced urothelial carcinoma.
Funding: Bristol-Myers Squibb.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
P Sharma reports receiving fees for advisory board participation for Jounce and Kite, fees for consultancy from Jounce, Kite, Bristol-Myers Squibb (BMS), AstraZeneca, and Amgen, and stock/stock options with Jounce and Kite.
MKC reports receiving grants from BMS, consultancy fees from AstraZeneca and Moderna, and payment for lectures from Clinical Care Options.
PB reports honoraria from BMS during the conduct of the study, honoraria from Pfizer, MSD, and Orion Pharma, and research funding from Novartis.
JK declares no competing interests.
P Spiliopoulou declares no competing interests.
E Calvo declares no competing interests.
RNP reports receiving a travel grant from BMS for an investigator meeting during the conduct of the study.
PAO reports receiving a grant from BMS and consultancy fees from BMS, Amgen, Celldex, Alexion, and Cytomx.
FdB reports receiving consultancy fees from Tiziana Life Sciences, BMS, MSD, Servier, Eli Lilly, Merck Serono, GlaxoSmithKline, and Novartis, and speaker fees from BMS, Eli Lilly, Roche, and ACCMED.
MM reports receiving consultancy fees from Etubics and Boehringer Ingelheim, and speaker fees from Genentech, Novartis, Sanofi, Regeneron, Lexicon, Ipsen, Onyx, Bayer, Taiho, Merrimack, and Celgene.
DTL declares no competing interests.
DJ reports being an employee of BMS.
E Chan reports receiving a grant from BMS for the conduct of the trial and consultancy fees for advisory board participation from EMD Serono, Taiho, Bayer, Advaxis, Amgen, Lilly, and Castle Biosciences.
CH reports being an employee of and holding stock options with BMS.
C-SL reports being an employee of and holding stock options with BMS.
MT reports being an employee of and holding stock options with BMS.
AA reports being an employee of BMS.
JER reports receiving a grant from BMS for the conduct of the study, consultancy fees from Roche/Genentech, AstraZeneca, Eli Lilly, Agensys, Sanofi US Services, Oncogenex, Onyx, Dendreon, BMS, and Boehringer Ingelheim, grants from Novartis and Roche/Genetech, and holding stock/stock options with Illumina and Merck.
Figures


Comment in
-
Immunomodulatory treatment in urothelial cancer.Lancet Oncol. 2016 Nov;17(11):1475-1477. doi: 10.1016/S1470-2045(16)30497-1. Epub 2016 Oct 9. Lancet Oncol. 2016. PMID: 27733242 No abstract available.
References
-
- Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8:1050–5. - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77. - PubMed
-
- Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30:1107–13. - PMC - PubMed
-
- Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. - PubMed
-
- Bellmunt J, Fougeray R, Rosenberg JE, et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol. 2013;24:1466–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

