Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials
- PMID: 27733360
- PMCID: PMC5062056
- DOI: 10.1136/bmj.i5044
Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials
Erratum in
-
Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomised controlled trials.BMJ. 2016 Nov 29;355:i6416. doi: 10.1136/bmj.i6416. BMJ. 2016. PMID: 27899362 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the effectiveness of antenatal corticosteroids given at ≥34 weeks' gestation.
Design: Systematic review with meta-analysis.
Data sources: Electronic databases were searched from their inception to February 2016.
Eligibility criteria for study selection: Randomized clinical trials comparing antenatal corticosteroids with placebo or no treatment in women with a singleton pregnancy at ≥34 weeks' gestation. Trials on antenatal steroids in women expected to deliver late preterm (340-366 weeks) and trials given before planned cesarean delivery at term (≥37 weeks) were included.
Data synthesis: The primary outcome was the incidence of severe respiratory distress syndrome (RDS). The summary measures were reported as relative risks or mean differences with 95% confidence intervals.
Results: Six trials, including 5698 singleton pregnancies, were analyzed. Three included 3200 women at 340-366 weeks' gestation and at risk of imminent premature delivery at the time of hospital admission. The three other trials included 2498 women undergoing planned cesarean delivery at ≥37 weeks. Overall, infants of mothers who received antenatal corticosteroids at ≥34 weeks had a significantly lower risk of RDS (relative risk 0.74, 95% confidence interval 0.61 to 0.91), mild RDS (0.67, 0.46 to 0.96), moderate RDS (0.39, 0.18 to 0.89), transient tachypnea of the newborn (0.56, 0.37 to 0.86), severe RDS (0.55, 0.33 to 0.91), use of surfactant, and mechanical ventilation, and a significantly lower time receiving oxygen (mean difference -2.06 hours, 95% confidence interval -2.17 to -1.95), lower maximum inspired oxygen concentration (-0.66%, -0.69% to -0.63%), shorter stay on a neonatal intensive care unit (-7.64 days, -7.65 to -7.64), and higher APGAR scores compared with controls. Infants of mothers who received antenatal betamethasone at 340-366 weeks' gestation had a significantly lower incidence of transient tachypnea of the newborn (relative risk 0.72, 95% confidence interval 0.56 to 0.92), severe RDS (0.60, 0.33 to 0.94), and use of surfactant (0.61, 0.38 to 0.99). Infants of mothers undergoing planned cesarean delivery at ≥37 weeks' gestation who received prophylactic antenatal corticosteroids 48 hours before delivery had a significantly lower risk of RDS (0.40, 0.27 to 0.59), mild RDS (0.43, 0.26 to 0.72), moderate RDS (0.40, 0.18 to 0.88), transient tachypnea of the newborn (0.38, 0.25 to 0.57), and mechanical ventilation (0.19, 0.08 to 0.43), and significantly less time receiving oxygen (mean difference -2.06 hours, 95% confidence interval -2.17 to -1.95), lower percentage of maximum inspired oxygen concentration (-0.66%, -0.69% to -0.63%), shorter stay in neonatal intensive care (-7.44 days, -7.44 to -7.43), and a higher APGAR score at one and at five minutes.
Conclusions: Antenatal steroids at ≥34 weeks' gestation reduce neonatal respiratory morbidity. A single course of corticosteroids can be considered for women at risk of imminent late premature delivery 340-366 weeks' gestation, as well as for women undergoing planned cesarean delivery at ≥37 weeks' gestation.
Systematic review registration: PROSPERO CRD42016035234.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


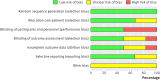


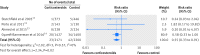
References
-
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep 2015;64:1-65.pmid:25603115. - PubMed
-
- Doyle LW. Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis. Pediatrics 2001;108:134-41. 10.1542/peds.108.1.134 pmid:11433066. - DOI - PubMed
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006;19:CD004454.pmid:16856047. - PubMed
-
- Royal College of Obstetricians and Gynaecologists (RCOG). Antenatal corticosteroids to prevent respiratory distress syndrome. Clinical Green Top Guidelines.Royal College of Obstetricians and Gynaecologists, 2004.
-
- Committee Opinion No. 677. ACOG committee opinion: antenatal corticosteroids therapy for fetal maturation. Obstet Gynecol 2016;128:e187-94. 10.1097/AOG.0000000000001715 pmid:27661658. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
