Phase II trial of post-operative radiotherapy with concurrent cisplatin plus panitumumab in patients with high-risk, resected head and neck cancer
- PMID: 27733374
- PMCID: PMC5178143
- DOI: 10.1093/annonc/mdw428
Phase II trial of post-operative radiotherapy with concurrent cisplatin plus panitumumab in patients with high-risk, resected head and neck cancer
Abstract
Background: Treatment intensification for resected, high-risk, head and neck squamous cell carcinoma (HNSCC) is an area of active investigation with novel adjuvant regimens under study. In this trial, the epidermal growth-factor receptor (EGFR) pathway was targeted using the IgG2 monoclonal antibody panitumumab in combination with cisplatin chemoradiotherapy (CRT) in high-risk, resected HNSCC.
Patients and methods: Eligible patients included resected pathologic stage III or IVA squamous cell carcinoma of the oral cavity, larynx, hypopharynx, or human-papillomavirus (HPV)-negative oropharynx, without gross residual tumor, featuring high-risk factors (margins <1 mm, extracapsular extension, perineural or angiolymphatic invasion, or ≥2 positive lymph nodes). Postoperative treatment consisted of standard RT (60-66 Gy over 6-7 weeks) concurrent with weekly cisplatin 30 mg/m2 and weekly panitumumab 2.5 mg/kg. The primary endpoint was progression-free survival (PFS).
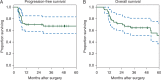
Results: Forty-six patients were accrued; 44 were evaluable and were analyzed. The median follow-up for patients without recurrence was 49 months (range 12-90 months). The probability of 2-year PFS was 70% (95% CI = 58%-85%), and the probability of 2-year OS was 72% (95% CI = 60%-87%). Fourteen patients developed recurrent disease, and 13 (30%) of them died. An additional five patients died from causes other than HNSCC. Severe (grade 3 or higher) toxicities occurred in 14 patients (32%).
Conclusions: Intensification of adjuvant treatment adding panitumumab to cisplatin CRT is tolerable and demonstrates improved clinical outcome for high-risk, resected, HPV-negative HNSCC patients. Further targeted monoclonal antibody combinations are warranted.
Registered clinical trial number: NCT00798655.
Keywords: cisplatin chemoradiotherapy; clinical trial; head and neck squamous cell carcinoma; panitumumab.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24(14): 2137–2150. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60(5): 277–300. - PubMed
-
- Bernier J, Domenge C, Ozsahin M et al. . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350(19): 1945–1952. - PubMed
-
- Cooper JS, Pajak TF, Forastiere AA et al. . Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350(19): 1937–1944. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous