Evaluating the safety and dosing of drugs in patients with liver cirrhosis by literature review and expert opinion
- PMID: 27733414
- PMCID: PMC5073492
- DOI: 10.1136/bmjopen-2016-012991
Evaluating the safety and dosing of drugs in patients with liver cirrhosis by literature review and expert opinion
Abstract
Introduction: Liver cirrhosis can have a major impact on drug pharmacokinetics and pharmacodynamics. Patients with cirrhosis often suffer from potentially preventable adverse drug reactions. Guidelines on safe prescribing for these patients are lacking. The aim of this study is to develop a systematic method for evaluating the safety and optimal dosage of drugs in patients with liver cirrhosis.
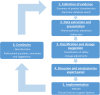
Methods and analysis: For each drug, a six-step evaluation process will be followed. (1) Available evidence on the pharmacokinetics and safety of a drug in patients with liver cirrhosis will be collected from the Summary of Product Characteristics (SmPC) and a systematic literature review will be performed. (2) Data regarding two outcomes, namely pharmacokinetics and safety, will be extracted and presented in a standardised assessment report. (3) A safety classification and dosage suggestion will be proposed for each drug. (4) An expert panel will discuss the validity and clinical relevance of this suggested advice. (5) Advices will be implemented in all relevant Clinical Decision Support Systems in the Netherlands and published on a website for patients and healthcare professionals. (6) The continuity of the advices will be guaranteed by a yearly check of new literature and comments on the advices. This protocol will be applied in the evaluation of a selection of drugs: (A) drugs used to treat (complications of) liver cirrhosis, and (B) drugs frequently prescribed to the general population.
Ethics and dissemination: Since this study does not directly involve human participants, it does not require ethical clearance. Besides implementation on a website and in clinical decision support systems, we aim to publish the generated advices of one or two drug classes in a peer-reviewed journal and at conference meetings.
Keywords: drug safety; expert opinion; literature review; liver cirrhosis; practice guideline.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
DB has received research grants from BMS, MSD and ViiV and has performed teaching for Abbvie, BMS, Gilead, MSD and ViiV, outside the submitted work. JD has received research grants from Abbvie and Janssen and has been a member of advisory boards of AbbVie, BMS, Gilead, Janssen, and Merck, outside the submitted work. HM has received research grants from AbbVie, Astellas, Novartis and Gilead and has been a member of advisory boards of AbbVie, Astellas and Novartis, outside the submitted work.
Figures
Similar articles
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis.Cochrane Database Syst Rev. 2016 Oct 4;10(10):CD011812. doi: 10.1002/14651858.CD011812.pub2. Cochrane Database Syst Rev. 2016. PMID: 27699783 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
The Burden of Inappropriate Prescriptions and Predictors for Hospitalized Patients with Liver Cirrhosis in Ethiopia.Hepat Med. 2023 Sep 25;15:129-140. doi: 10.2147/HMER.S423351. eCollection 2023. Hepat Med. 2023. PMID: 37790886 Free PMC article.
-
Improving Medication-Related Outcomes in Chronic Liver Disease.Hepatol Commun. 2020 Oct 10;4(11):1562-1577. doi: 10.1002/hep4.1612. eCollection 2020 Nov. Hepatol Commun. 2020. PMID: 33163829 Free PMC article. Review.
-
Drug Treatment of Patients with Liver Cirrhosis in a Tertiary Hospital in Northern Ghana: Does It Comply with Recommended Guidelines?Int J Hepatol. 2020 May 28;2020:9750194. doi: 10.1155/2020/9750194. eCollection 2020. Int J Hepatol. 2020. PMID: 32550025 Free PMC article.
-
Recommendations for the safe use of direct oral anticoagulants in patients with cirrhosis based on a systematic review of pharmacokinetic, pharmacodynamic and safety data.Eur J Clin Pharmacol. 2024 Jun;80(6):797-812. doi: 10.1007/s00228-024-03648-y. Epub 2024 Mar 2. Eur J Clin Pharmacol. 2024. PMID: 38430266
-
Statin-Induced Rhabdomyolysis Associated With Transjugular Intrahepatic Portosystemic Shunt Placement.ACG Case Rep J. 2022 May 4;9(5):e00774. doi: 10.14309/crj.0000000000000774. eCollection 2022 May. ACG Case Rep J. 2022. PMID: 35919670 Free PMC article.
References
-
- Food and Drug Administration. Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. Updated 2003. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical