Learned motivation drives circadian physiology in the absence of the master circadian clock
- PMID: 27733449
- PMCID: PMC5161517
- DOI: 10.1096/fj.201600926R
Learned motivation drives circadian physiology in the absence of the master circadian clock
Abstract
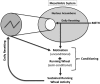
The suprachiasmatic nucleus (SCN)-often referred to as the master circadian clock-is essential in generating physiologic rhythms and orchestrating synchrony among circadian clocks. This study tested the hypothesis that periodic motivation induced by rhythmically pairing 2 reinforcing stimuli [methamphetamine (Meth) and running wheel (RW)] restores autonomous circadian activity in arrhythmic SCN-lesioned (SCNX) C3H/HeN mice. Sham-surgery and SCNX mice were treated with either Meth (1.2 mg/kg, i.p.) or vehicle in association, dissociation, or absence of an RW. Only the association of Meth treatment and restricted RW access successfully reestablished entrained circadian rhythms in mice with SCNX. RW-likely acting as a link between the circadian and reward systems-promotes circadian entrainment of activity. We conclude that a conditioned drug response is a powerful tool to entrain, drive, and restore circadian physiology. Furthermore, an RW should be recognized as a potent input signal in addition to the conventional use as an output signal.-Rawashdeh, O., Clough, S. J., Hudson, R. L., Dubocovich, M. L. Learned motivation drives circadian physiology in the absence of the master circadian clock.
Keywords: SCN lesions; methamphetamine; running wheel.
© FASEB.
Figures





References
-
- Roenneberg T., Daan S., Merrow M. (2003) The art of entrainment. J. Biol. Rhythms 18, 183–194 - PubMed
-
- Dibner C., Schibler U., Albrecht U. (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 - PubMed
-
- Mistlberger R. E. (2005) Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Brain Res. Rev. 49, 429–454 - PubMed
-
- Ibata Y., Okamura H., Tanaka M., Tamada Y., Hayashi S., Iijima N., Matsuda T., Munekawa K., Takamatsu T., Hisa Y., Shigeyoshi Y., Amaya F. (1999) Functional morphology of the suprachiasmatic nucleus. Front. Neuroendocrinol. 20, 241–268 - PubMed
-
- Kalsbeek A., Palm I. F., La Fleur S. E., Scheer F. A., Perreau-Lenz S., Ruiter M., Kreier F., Cailotto C., Buijs R. M. (2006) SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 21, 458–469 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

