Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells
- PMID: 27733558
- PMCID: PMC5500303
- DOI: 10.1126/scitranslmed.aaf9336
Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells
Abstract
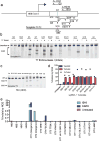
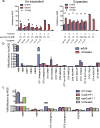
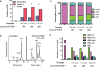
Genetic diseases of blood cells are prime candidates for treatment through ex vivo gene editing of CD34+ hematopoietic stem/progenitor cells (HSPCs), and a variety of technologies have been proposed to treat these disorders. Sickle cell disease (SCD) is a recessive genetic disorder caused by a single-nucleotide polymorphism in the β-globin gene (HBB). Sickle hemoglobin damages erythrocytes, causing vasoocclusion, severe pain, progressive organ damage, and premature death. We optimize design and delivery parameters of a ribonucleoprotein (RNP) complex comprising Cas9 protein and unmodified single guide RNA, together with a single-stranded DNA oligonucleotide donor (ssODN), to enable efficient replacement of the SCD mutation in human HSPCs. Corrected HSPCs from SCD patients produced less sickle hemoglobin RNA and protein and correspondingly increased wild-type hemoglobin when differentiated into erythroblasts. When engrafted into immunocompromised mice, ex vivo treated human HSPCs maintain SCD gene edits throughout 16 weeks at a level likely to have clinical benefit. These results demonstrate that an accessible approach combining Cas9 RNP with an ssODN can mediate efficient HSPC genome editing, enables investigator-led exploration of gene editing reagents in primary hematopoietic stem cells, and suggests a path toward the development of new gene editing treatments for SCD and other hematopoietic diseases.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures

References
-
- Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatr Blood Cancer. 2012;59:377–85. - PubMed
-
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle Cell Anemia, a Molecular Disease. Science (80-) 1949;110:543–548. - PubMed
-
- Neel JV. The Inheritance of Sickle Cell Anemia. Science. 1949;110:64–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

