Cell-autonomous stress responses in innate immunity
- PMID: 27733577
- PMCID: PMC6608034
- DOI: 10.1189/jlb.2MR0416-201R
Cell-autonomous stress responses in innate immunity
Abstract
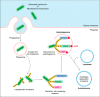
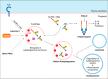
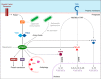
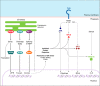
The innate immune response of phagocytes to microbes has long been known to depend on the core signaling cascades downstream of pattern recognition receptors (PRRs), which lead to expression and production of inflammatory cytokines that counteract infection and induce adaptive immunity. Cell-autonomous responses have recently emerged as important mechanisms of innate immunity. Either IFN-inducible or constitutive, these processes aim to guarantee cell homeostasis but have also been shown to modulate innate immune response to microbes and production of inflammatory cytokines. Among these constitutive cell-autonomous responses, autophagy is prominent and its role in innate immunity has been well characterized. Other stress responses, such as metabolic stress, the ER stress/unfolded protein response, mitochondrial stress, or the DNA damage response, seem to also be involved in innate immunity, although the precise mechanisms by which they regulate the innate immune response are not yet defined. Of importance, these distinct constitutive cell-autonomous responses appear to be interconnected and can also be modulated by microbes and PRRs, which add further complexity to the interplay between innate immune signaling and cell-autonomous responses in the mediation of an efficient innate immune response.
Keywords: DNA damage response; ER stress/UPR; autophagy; mTOR; mitochondrial stress; stress granules.
© Society for Leukocyte Biology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

