Clarithromycin increases neuronal excitability in CA3 pyramidal neurons through a reduction in GABAergic signaling
- PMID: 27733592
- PMCID: PMC5209546
- DOI: 10.1152/jn.00134.2016
Clarithromycin increases neuronal excitability in CA3 pyramidal neurons through a reduction in GABAergic signaling
Abstract
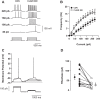
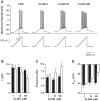
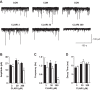
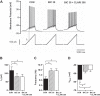
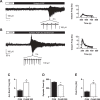
Antibiotics are used in the treatment and prevention of bacterial infections, but effects on neuron excitability have been documented. A recent study demonstrated that clarithromycin alleviates daytime sleepiness in hypersomnia patients (Trotti LM, Saini P, Freeman AA, Bliwise DL, García PS, Jenkins A, Rye DB. J Psychopharmacol 28: 697-702, 2014). To explore the potential application of clarithromycin as a stimulant, we performed whole cell patch-clamp recordings in rat pyramidal cells from the CA3 region of hippocampus. In the presence of the antibiotic, rheobase current was reduced by 50%, F-I relationship (number of action potentials as a function of injected current) was shifted to the left, and the resting membrane potential was more depolarized. Clarithromycin-induced hyperexcitability was dose dependent; doses of 30 and 300 μM clarithromycin significantly increased the firing frequency and membrane potential compared with controls (P = 0.003, P < 0.0001). We hypothesized that clarithromycin enhanced excitability by reducing GABAA receptor activation. Clarithromycin at 30 μM significantly reduced (P = 0.001) the amplitude of spontaneous miniature inhibitory GABAergic currents and at 300 μM had a minor effect on action potential width. Additionally, we tested the effect of clarithromycin in an ex vivo seizure model by evaluating its effect on spontaneous local field potentials. Bath application of 300 μM clarithromycin enhanced burst frequency twofold compared with controls (P = 0.0006). Taken together, these results suggest that blocking GABAergic signaling with clarithromycin increases cellular excitability and potentially serves as a stimulant, facilitating emergence from anesthesia or normalizing vigilance in hypersomnia and narcolepsy. However, the administration of clarithromycin should be carefully considered in patients with seizure disorders.
New & noteworthy: Clinical administration of the macrolide antibiotic clarithromycin has been associated with side effects such as mania, agitation, and delirium. Here, we investigated the adverse effects of this antibiotic on CA3 pyramidal cell excitability. Clarithromycin induces hyperexcitability in single neurons and is related to a reduction in GABAergic signaling. Our results support a potentially new application of clarithromycin as a stimulant to facilitate emergence from anesthesia or to normalize vigilance.
Keywords: GABAA receptor; clarithromycin; hippocampus; neuronal excitability.
Figures


References
-
- Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol 22: 71–81, 2002. - PubMed
-
- Bhattacharyya S, Darby RR, Raibagkar P, Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology 86: 963–971, 2016. - PubMed
-
- Chu SY, Sennello LT, Sonders RC. Simultaneous determination of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr 571: 199–208, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

