Neural Stem Cell Transplantation Induces Stroke Recovery by Upregulating Glutamate Transporter GLT-1 in Astrocytes
- PMID: 27733606
- PMCID: PMC5059427
- DOI: 10.1523/JNEUROSCI.1643-16.2016
Neural Stem Cell Transplantation Induces Stroke Recovery by Upregulating Glutamate Transporter GLT-1 in Astrocytes
Abstract
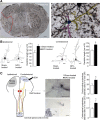
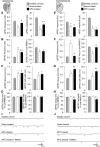
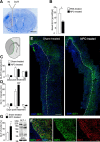
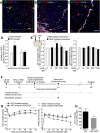
Ischemic stroke is the leading cause of disability, but effective therapies are currently widely lacking. Recovery from stroke is very much dependent on the possibility to develop treatments able to both halt the neurodegenerative process as well as to foster adaptive tissue plasticity. Here we show that ischemic mice treated with neural precursor cell (NPC) transplantation had on neurophysiological analysis, early after treatment, reduced presynaptic release of glutamate within the ipsilesional corticospinal tract (CST), and an enhanced NMDA-mediated excitatory transmission in the contralesional CST. Concurrently, NPC-treated mice displayed a reduced CST degeneration, increased axonal rewiring, and augmented dendritic arborization, resulting in long-term functional amelioration persisting up to 60 d after ischemia. The enhanced functional and structural plasticity relied on the capacity of transplanted NPCs to localize in the peri-ischemic and ischemic area, to promote the upregulation of the glial glutamate transporter 1 (GLT-1) on astrocytes and to reduce peri-ischemic extracellular glutamate. The upregulation of GLT-1 induced by transplanted NPCs was found to rely on the secretion of VEGF by NPCs. Blocking VEGF during the first week after stroke reduced GLT-1 upregulation as well as long-term behavioral recovery in NPC-treated mice. Our results show that NPC transplantation, by modulating the excitatory-inhibitory balance and stroke microenvironment, is a promising therapy to ameliorate disability, to promote tissue recovery and plasticity processes after stroke.
Significance statement: Tissue damage and loss of function occurring after stroke can be constrained by fostering plasticity processes of the brain. Over the past years, stem cell transplantation for repair of the CNS has received increasing interest, although underlying mechanism remain elusive. We here show that neural stem/precursor cell transplantation after ischemic stroke is able to foster axonal rewiring and dendritic plasticity and to induce long-term functional recovery. The observed therapeutic effect of neural precursor cells seems to underlie their capacity to upregulate the glial glutamate transporter on astrocytes through the vascular endothelial growth factor inducing favorable changes in the electrical and molecular stroke microenvironment. Cell-based approaches able to influence plasticity seem particularly suited to favor poststroke recovery.
Keywords: ischemia; neurophysiology; plasticity; recovery; stem cell; transplantation.
Copyright © 2016 Bacigaluppi et al.
Figures





References
-
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. - DOI - PMC - PubMed
-
- Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. - DOI - PubMed
-
- Bernhardt J, Langhorne P, Lindley RI, Thrift AG, Ellery F, Collier J, Churilov L, Moodie M, Dewey H, Donnan G. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015;386:46–55. doi: 10.1016/S0140-6736(15)60690-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
