Modafinil Activates Phasic Dopamine Signaling in Dorsal and Ventral Striata
- PMID: 27733628
- PMCID: PMC5118647
- DOI: 10.1124/jpet.116.236000
Modafinil Activates Phasic Dopamine Signaling in Dorsal and Ventral Striata
Abstract
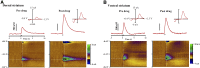
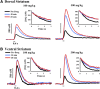
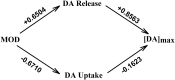
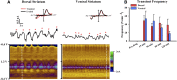
Modafinil (MOD) exhibits therapeutic efficacy for treating sleep and psychiatric disorders; however, its mechanism is not completely understood. Compared with other psychostimulants inhibiting dopamine (DA) uptake, MOD weakly interacts with the dopamine transporter (DAT) and modestly elevates striatal dialysate DA, suggesting additional targets besides DAT. However, the ability of MOD to induce wakefulness is abolished with DAT knockout, conversely suggesting that DAT is necessary for MOD action. Another psychostimulant target, but one not established for MOD, is activation of phasic DA signaling. This communication mode during which burst firing of DA neurons generates rapid changes in extracellular DA, the so-called DA transients, is critically implicated in reward learning. Here, we investigate MOD effects on phasic DA signaling in the striatum of urethane-anesthetized rats with fast-scan cyclic voltammetry. We found that MOD (30-300 mg/kg i.p.) robustly increases the amplitude of electrically evoked phasic-like DA signals in a time- and dose-dependent fashion, with greater effects in dorsal versus ventral striata. MOD-induced enhancement of these electrically evoked amplitudes was mediated preferentially by increased DA release compared with decreased DA uptake. Principal component regression of nonelectrically evoked recordings revealed negligible changes in basal DA with high-dose MOD (300 mg/kg i.p.). Finally, in the presence of the D2 DA antagonist, raclopride, low-dose MOD (30 mg/kg i.p.) robustly elicited DA transients in dorsal and ventral striata. Taken together, these results suggest that activation of phasic DA signaling is an important mechanism underlying the clinical efficacy of MOD.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




References
-
- Anderson DR. (2008) Model Based Inference in the Life Sciences. A Primer on Evidence, Springer Science Business Media, New York.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

