Xpert MTB/RIF Assay Shows Faster Clearance of Mycobacterium tuberculosis DNA with Higher Levels of Rifapentine Exposure
- PMID: 27733634
- PMCID: PMC5121396
- DOI: 10.1128/JCM.01313-16
Xpert MTB/RIF Assay Shows Faster Clearance of Mycobacterium tuberculosis DNA with Higher Levels of Rifapentine Exposure
Abstract
The Xpert MTB/RIF assay is both sensitive and specific as a diagnostic test. Xpert also reports quantitative output in cycle threshold (CT) values, which may provide a dynamic measure of sputum bacillary burden when used longitudinally. We evaluated the relationship between Xpert CT trajectory and drug exposure during tuberculosis (TB) treatment to assess the potential utility of Xpert CT for treatment monitoring. We obtained serial sputum samples from patients with smear-positive pulmonary TB who were consecutively enrolled at 10 international clinical trial sites participating in study 29X, a CDC-sponsored Tuberculosis Trials Consortium study evaluating the tolerability, safety, and antimicrobial activity of rifapentine at daily doses of up to 20 mg/kg of body weight. Xpert was performed at weeks 0, 2, 4, 6, 8, and 12. Longitudinal CT data were modeled using a nonlinear mixed effects model in relation to rifapentine exposure (area under the concentration-time curve [AUC]). The rate of change of CT was higher in subjects receiving rifapentine than in subjects receiving standard-dose rifampin. Moreover, rifapentine exposure, but not assigned dose, was significantly associated with rate of change in CT (P = 0.02). The estimated increase in CT slope for every additional 100 μg · h/ml of rifapentine drug exposure (as measured by AUC) was 0.11 CT/week (95% confidence interval [CI], 0.05 to 0.17). Increasing rifapentine exposure is associated with a higher rate of change of Xpert CT, indicating faster clearance of Mycobacterium tuberculosis DNA. These data suggest that the quantitative outputs of the Xpert MTB/RIF assay may be useful as a dynamic measure of TB treatment response.
Copyright © 2016 Jayakumar et al.
Figures
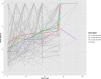
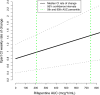
References
-
- Nahid P, Saukkonen J, Mac Kenzie WR, Johnson JL, Phillips PP, Andersen J, Bliven-Sizemore E, Belisle JT, Boom WH, Luetkemeyer A, Campbell TB, Eisenach KD, Hafner R, Lennox JL, Makhene M, Swindells S, Villarino ME, Weiner M, Benson C, Burman W, National Institutes of Health, Centers for Disease Control and Prevention. 2011. CDC/NIH workshop. Tuberculosis biomarker and surrogate endpoint research roadmap. Am J Respir Crit Care Med 184:972–979. - PMC - PubMed
-
- World Health Organization. 2016. TB diagnostics and laboratory strengthening. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

