Glycine 105 as Pivot for a Critical Knee-like Joint between Cytoplasmic and Transmembrane Segments of the Second Transmembrane Helix in Ca2+-ATPase
- PMID: 27733680
- PMCID: PMC5114418
- DOI: 10.1074/jbc.M116.759704
Glycine 105 as Pivot for a Critical Knee-like Joint between Cytoplasmic and Transmembrane Segments of the Second Transmembrane Helix in Ca2+-ATPase
Abstract
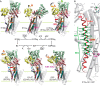
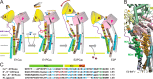
The cytoplasmic actuator domain of the sarco(endo)plasmic reticulum Ca2+-ATPase undergoes large rotational movements that influence the distant transmembrane transport sites, and a long second transmembrane helix (M2) connected with this domain plays critical roles in transmitting motions between the cytoplasmic catalytic domains and transport sites. Here we explore possible structural roles of Gly105 between the cytoplasmic (M2c) and transmembrane (M2m) segments of M2 by introducing mutations that limit/increase conformational freedom. Alanine substitution G105A markedly retards isomerization of the phosphoenzyme intermediate (E1PCa2 → E2PCa2 → E2P + 2Ca2+), and disrupts Ca2+ occlusion in E1PCa2 and E2PCa2 at the transport sites uncoupling ATP hydrolysis and Ca2+ transport. In contrast, this substitution accelerates the ATPase activation (E2 → E1Ca2). Introducing a glycine by substituting another residue on M2 in the G105A mutant (i.e. "G-shift substitution") identifies the glycine positions required for proper Ca2+ handling and kinetics in each step. All wild-type kinetic properties, including coupled transport, are fully restored in the G-shift substitution at position 112 (G105A/A112G) located on the same side of the M2c helix as Gly105 facing M4/phosphorylation domain. Results demonstrate that Gly105 functions as a flexible knee-like joint during the Ca2+ transport cycle, so that cytoplasmic domain motions can bend and strain M2 in the correct direction or straighten the helix for proper gating and coupling of Ca2+ transport and ATP hydrolysis.
Keywords: calcium ATPase; domain motion; enzyme kinetics; enzyme mechanism; enzyme mutation; enzyme structure; glycine; phosphoenzyme intermediate; sarco(endo)plasmic reticulum; transmembrane helix.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Toyoshima C. (2008) Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 476, 3–11 - PubMed
-
- Toyoshima C. (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 1793, 941–946 - PubMed
-
- Møller J. V., Olesen C., Winther A.-M. L., and Nissen P. (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 - PubMed
-
- Toyoshima C., Nakasako M., Nomura H., and Ogawa H. (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 - PubMed
-
- Danko S., Yamasaki K., Daiho T., Suzuki H., and Toyoshima C. (2001) Organization of cytoplasmic domains of sarcoplasmic reticulum Ca2+-ATPase in E1P and E1ATP states: a limited proteolysis study. FEBS Lett. 505, 129–135 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

