Effects of parathyroid hormone rhPTH(1-84) on phosphate homeostasis and vitamin D metabolism in hypoparathyroidism: REPLACE phase 3 study
- PMID: 27734257
- PMCID: PMC5225224
- DOI: 10.1007/s12020-016-1141-0
Effects of parathyroid hormone rhPTH(1-84) on phosphate homeostasis and vitamin D metabolism in hypoparathyroidism: REPLACE phase 3 study
Abstract
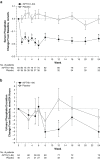
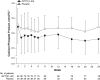
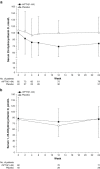
In hypoparathyroidism, inappropriately low levels of parathyroid hormone lead to unbalanced mineral homeostasis. The objective of this study was to determine the effect of recombinant human parathyroid hormone, rhPTH(1-84), on phosphate and vitamin D metabolite levels in patients with hypoparathyroidism. Following pretreatment optimization of calcium and vitamin D doses, 124 patients in a phase III, 24-week, randomized, double-blind, placebo-controlled study of adults with hypoparathyroidism received subcutaneous injections of placebo or rhPTH(1-84) (50 µg/day, titrated to 75 and then 100 µg/day, to permit reductions in oral calcium and active vitamin D doses while maintaining serum calcium within 2.0-2.2 mmol/L). Predefined endpoints related to phosphate homeostasis and vitamin D metabolism were analyzed. Serum phosphate levels decreased rapidly from the upper normal range and remained lower with rhPTH(1-84) (P < 0.001 vs. placebo). At week 24, serum calcium-phosphate product was lower with rhPTH(1-84) vs. placebo (P < 0.001). rhPTH(1-84) treatment resulted in significant reductions in oral calcium dose compared with placebo (P < 0.001) while maintaining serum calcium. After pretreatment optimization, baseline serum 25-hydroxyvitamin D (25[OH]D) and 1,25-dihydroxyvitamin D (1,25[OH]2D) levels were within the normal range in both groups. After 24 weeks, 1,25(OH)2D levels were unchanged in both treatment groups, despite significantly greater reductions in active vitamin D dose in the rhPTH(1-84) group. In hypoparathyroidism, rhPTH(1-84) reduces serum phosphate levels, improves calcium-phosphate product, and maintains 1,25(OH)2D and serum calcium in the normal range while allowing significant reductions in active vitamin D and oral calcium doses.
Keywords: Hypoparathyroidism; Parathyroid hormone; Phosphate; Vitamin D; rhPTH(1–84).
Conflict of interest statement
BLC, JPB, and DMS have received institutional research grants from and served as advisory group members for NPS Pharmaceuticals, Inc.; MM and TJV have served as advisory group members for NPS Pharmaceuticals, Inc.; HL was an employee of NPS Pharmaceuticals, Inc., at the time the study was conducted and the manuscript was written. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Bohrer T, Pasteur I, Lyutkevych O, Fleischmann P, Sitter H, Donner-Banzhoff N, Rybakov S, Hofmann C, Tronko M, Rothmund M. Permanent postoperative hypoparathyroidism. An epidemiological clinical study using a new questionnaire instrument. J. Ukrainian Acad. Sci. 2003;9:476–494.
-
- National Organization for Rare Disorders, Hypoparathyroidism. (2014) http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/.... Accessed 15 September 2016
-
- Potts JT., Jr . Disorders of the parathyroid gland and calcium homeostasis. In: Longo DL, K. D, Jameson JL, Fauci AS, Hauser SL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 18th Edn. New York: McGraw-Hill; 2012. pp. 3096–3120.
-
- Bilezikian JP, Khan A, Potts JT, Jr., Brandi ML, Clarke BL, Shoback D, Juppner H, D’Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J. Bone Miner. Res. 2011;26(10):2317–2337. doi: 10.1002/jbmr.483. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

