The evolution of Ebola virus: Insights from the 2013-2016 epidemic
- PMID: 27734858
- PMCID: PMC5580494
- DOI: 10.1038/nature19790
The evolution of Ebola virus: Insights from the 2013-2016 epidemic
Abstract
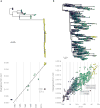
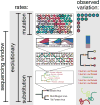
The 2013-2016 epidemic of Ebola virus disease in West Africa was of unprecedented magnitude and changed our perspective on this lethal but sporadically emerging virus. This outbreak also marked the beginning of large-scale real-time molecular epidemiology. Here, we show how evolutionary analyses of Ebola virus genome sequences provided key insights into virus origins, evolution and spread during the epidemic. We provide basic scientists, epidemiologists, medical practitioners and other outbreak responders with an enhanced understanding of the utility and limitations of pathogen genomic sequencing. This will be crucially important in our attempts to track and control future infectious disease outbreaks.
Figures


References
-
- Baize S, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–1425. The first paper to describe the emergence of EBOV Makona in Guinea in December 2013, providing the sequences of three full-length viral genomes. - PubMed
-
- Gire SK, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345:1369–1372. Obtained the first large-scale near-real-time EBOV genomic data from 78 patients in Sierra Leone, which provided critical insights into virus spread during the early stages of the epidemic. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

