Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression - implications for atherosclerosis research
- PMID: 27734926
- PMCID: PMC5062347
- DOI: 10.1038/srep35234
Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression - implications for atherosclerosis research
Abstract
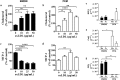
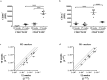
Macrophages are heterogeneous and can polarize into specific subsets, e.g. pro-inflammatory M1-like and re-modelling M2-like macrophages. To determine if peritoneal macrophages (PEMs) or bone marrow derived macrophages (BMDMs) resembled aortic macrophages from ApoE-/- mice, their M1/M2 phenotype, inflammatory status, and lipid metabolism signatures were compared. oxLDL accumulation was similar in PEMs and BMDMs. On protein expression level, BMDMs showed an M2-like CD206highCD11clow profile, while cholesterol loading led to enhanced CD11c expression and reduced MCP-1 secretion. In contrast, PEMs expressed low levels of CD206 and CD11c, and responded to cholesterol loading by increasing CD11c expression and MCP-1 secretion. mRNA expression of M1/M2 markers was higher in PEMS than BMDMs, while lipid metabolism genes were similarly expressed. Whole aorta flow cytometry showed an accumulation of M2-like CD206highCD11clow macrophages in advanced versus early atherosclerotic disease in ApoE-/- mice. In isolated lesions, mRNA levels of the M2 markers Socs2, CD206, Retnla, and IL4 were downregulated with increasing disease severity. Likewise, mRNA expression of lipid metabolism genes (SREBP2, ACSL1, SRB1, DGAT1, and cpt1a) was decreased in advanced versus early lesions. In conclusion, PEMs and BMDMs are phenotypically distinct and differ from macrophages in lesions with respect to expression of M1/M2 markers and lipid metabolism genes.
Conflict of interest statement
L.S.B., S.E.R., C.K.M., A.R. and H.C. has been or are employed at Novo Nordisk.
Figures

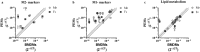
References
-
- Pillai M. M., Hayes B. & Torok-Storb B. Inducible transgenes under the control of the hCD68 promoter identifies mouse macrophages with a distribution that differs from the F4/80 - and CSF-1R-expressing populations. Exp Hematol 37, 1387–1392, doi: 10.1016/j.exphem.2009.09.003 (2009). - DOI - PMC - PubMed
-
- Balbo P., Silvestri M., Rossi G. A., Crimi E. & Burastero S. E. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy 31, 625–636 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

