Dual-targeting anti-angiogenic cyclic peptides as potential drug leads for cancer therapy
- PMID: 27734947
- PMCID: PMC5062114
- DOI: 10.1038/srep35347
Dual-targeting anti-angiogenic cyclic peptides as potential drug leads for cancer therapy
Abstract
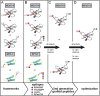
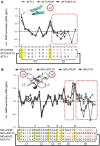
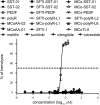
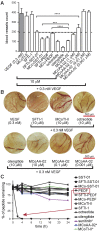
Peptide analogues derived from bioactive hormones such as somatostatin or certain growth factors have great potential as angiogenesis inhibitors for cancer applications. In an attempt to combat emerging drug resistance many FDA-approved anti-angiogenesis therapies are co-administered with cytotoxic drugs as a combination therapy to target multiple signaling pathways of cancers. However, cancer therapies often encounter limiting factors such as high toxicities and side effects. Here, we combined two anti-angiogenic epitopes that act on different pathways of angiogenesis into a single non-toxic cyclic peptide framework, namely MCoTI-II (Momordica cochinchinensis trypsin inhibitor-II), and subsequently assessed the anti-angiogenic activity of the novel compound. We hypothesized that the combination of these two epitopes would elicit a synergistic effect by targeting different angiogenesis pathways and result in improved potency, compared to that of a single epitope. This novel approach has resulted in the development of a potent, non-toxic, stable and cyclic analogue with nanomolar potency inhibition in in vitro endothelial cell migration and in vivo chorioallantoic membrane angiogenesis assays. This is the first report to use the MCoTI-II framework to develop a 2-in-1 anti-angiogenic peptide, which has the potential to be used as a form of combination therapy for targeting a wide range of cancers.
Figures


References
-
- Carmeliet P. & Jain R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000). - PubMed
-
- Singh M. & Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat. Biotechnol. 30, 648–657 (2012). - PubMed
-
- Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 320, 130–137 (2012). - PubMed
-
- Thornton A. D., Ravn P., Winslet M. & Chester K. Angiogenesis inhibition with bevacizumab and the surgical management of colorectal cancer. Br. J. Surg. 93, 1456–1463 (2006). - PubMed
-
- Chua T. C. Gastrointestinal oncological surgery in patients with metastatic cancer treated with angiogenesis inhibitors: safe or not? ANZ J. Surg. 79, 672–673 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

