Photoperiod induced obesity in the Brandt's vole (Lasiopodomys brandtii): a model of 'healthy obesity'?
- PMID: 27736740
- PMCID: PMC5117229
- DOI: 10.1242/dmm.026070
Photoperiod induced obesity in the Brandt's vole (Lasiopodomys brandtii): a model of 'healthy obesity'?
Abstract
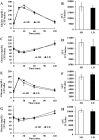
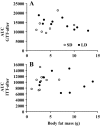
Brandt's voles have an annual cycle of body weight and adiposity. These changes can be induced in the laboratory by manipulation of photoperiod. In the present study, male captive-bred Brandt's voles aged 35 days were acclimated to a short day (SD) photoperiod (8L:16D) for 70 days. A subgroup of individuals (n=16) were implanted with transmitters to monitor physical activity and body temperature. They were then randomly allocated into long day (LD=16L:8D) (n=19, 8 with transmitters) and SD (n=18, 8 with transmitters) groups for an additional 70 days. We monitored aspects of energy balance, glucose and insulin tolerance (GTT and ITT), body composition and organ fat content after exposure to the different photoperiods. LD voles increased in weight for 35 days and then re-established stability at a higher level. At the end of the experiment LD-exposed voles had greater white adipose tissue mass than SD voles (P=0.003). During weight gain they did not differ in their food intake or digestive efficiency; however, daily energy expenditure was significantly reduced in the LD compared with SD animals (ANCOVA, P<0.05) and there was a trend to reduced resting metabolic rate RMR (P=0.075). Physical activity levels were unchanged. Despite different levels of fat storage, the GTT and ITT responses of SD and LD voles were not significantly different, and these traits were not correlated to body fatness. Hence, the photoperiod-induced obesity was independent on disruptions to glucose homeostasis, indicating a potential adaptive decoupling of these states in evolutionary time. Fat content in both the liver and muscle showed no significant difference between LD and SD animals. How voles overcome the common negative aspects of fat storage might make them a useful model for understanding the phenomenon of 'healthy obesity'.
Keywords: Adipose tissue expandability; Brandt's vole; Glucose tolerance; Healthy obesity; Insulin sensitivity; Lipotoxicity; Photoperiod.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





References
-
- Barroso I., Gurnell M., Crowley V. E. F., Agostini M., Schwabe J. W., Soos M. A., Maslen G. L., Williams T. D., Lewis H., Schafer A. J. et al. (1999). Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402, 880-883. - PubMed
-
- Bartness T. J., Demas G. E. and Song C. K. (2002). Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp. Biol. Med. 227, 363-376. - PubMed
-
- Butler P. J., Green J. A., Boyd I. L. and Speakman J. R. (2004). Measuring metabolic rate in the field: the pros and cons of the doubly-labelled water and heart rate methods. Funct. Ecol. 18, 168-183. 10.1111/j.0269-8463.2004.00821.x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

