Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa
- PMID: 27736756
- PMCID: PMC5119021
- DOI: 10.1128/AAC.01405-16
Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa
Abstract
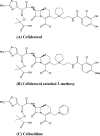
Cefiderocol (S-649266) is a novel parenteral siderophore cephalosporin conjugated with a catechol moiety at the third-position side chain. The in vitro activity of cefiderocol against Pseudomonas aeruginosa was enhanced under iron-depleted conditions, whereas that of ceftazidime was not affected. The monitoring of [thiazole-14C]cefiderocol revealed the increased intracellular accumulation of cefiderocol in P. aeruginosa cells incubated under iron-depleted conditions compared with those incubated under iron-sufficient conditions. Cefiderocol was shown to have potent chelating activity with ferric iron, and extracellular iron was efficiently transported into P. aeruginosa cells in the presence of cefiderocol as well as siderophores, while enhanced transport of extracellular ferric iron was not observed when one of the hydroxyl groups of the catechol moiety of cefiderocol was replaced with a methoxy group. We conclude that cefiderocol forms a chelating complex with iron, which is actively transported into P. aeruginosa cells via iron transporters, resulting in potent antibacterial activity of cefiderocol against P. aeruginosa.
Copyright © 2016 Ito et al.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

