Interleukin-17A (IL-17A) and IL-17F Are Critical for Antimicrobial Peptide Production and Clearance of Staphylococcus aureus Nasal Colonization
- PMID: 27736775
- PMCID: PMC5116734
- DOI: 10.1128/IAI.00596-16
Interleukin-17A (IL-17A) and IL-17F Are Critical for Antimicrobial Peptide Production and Clearance of Staphylococcus aureus Nasal Colonization
Abstract
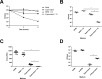
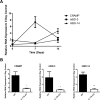
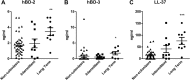
Approximately 20% of the population is persistently colonized by Staphylococcus aureus in the nares. Th17-like immune responses mediated by the interleukin-17 (IL-17) family of cytokines and neutrophils are becoming recognized as relevant host defense mechanisms for resolution of S. aureus mucocutaneous infections. Since antimicrobial peptides are regulated by the IL-17 cytokines, we sought to determine the role of IL-17 cytokines in production of antimicrobial peptides in a murine model of S. aureus nasal carriage. We discovered that nasal tissue supernatants have antistaphylococcal activity, and mice deficient in both IL-17A and IL-17F lost the ability to clear S. aureus nasal colonization. IL-17A was found to be sufficient for nasal mBD-3 production ex vivo and was required for CRAMP, mBD-3, and mBD-14 expression in response to S. aureus colonization in vivo These data were confirmed in a clinical study of nasal secretions in which elevated levels of the human forms of these antimicrobial peptides were found in nasal secretions from healthy human subjects when they were colonized with S. aureus but not in secretions from noncolonized subjects. Together, these data provide evidence for the importance of IL-17A regulation of antimicrobial peptides and IL-17F in the clearance of S. aureus nasal carriage.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Lehmann J, Retz M, Harder J, Krams M, Kellner U, Hartmann J, Hohgrawe K, Raffenberg U, Gerber M, Loch T, Weichert-Jacobsen K, Stockle M. 2002. Expression of human beta-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect Dis 2:20. doi: 10.1186/1471-2334-2-20. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

