Projected Uptake of New Antiretroviral (ARV) Medicines in Adults in Low- and Middle-Income Countries: A Forecast Analysis 2015-2025
- PMID: 27736953
- PMCID: PMC5063297
- DOI: 10.1371/journal.pone.0164619
Projected Uptake of New Antiretroviral (ARV) Medicines in Adults in Low- and Middle-Income Countries: A Forecast Analysis 2015-2025
Abstract
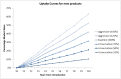
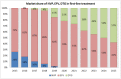
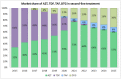
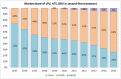
With anti-retroviral treatment (ART) scale-up set to continue over the next few years it is of key importance that manufacturers and planners in low- and middle-income countries (LMICs) hardest hit by the HIV/AIDS pandemic are able to anticipate and respond to future changes to treatment regimens, generics pipeline and demand, in order to secure continued access to all ARV medicines required. We did a forecast analysis, using secondary WHO and UNAIDS data sources, to estimate the number of people living with HIV (PLHIV) and the market share and demand for a range of new and existing ARV drugs in LMICs up to 2025. UNAIDS estimates 24.7 million person-years of ART in 2020 and 28.5 million person-years of ART in 2025 (24.3 million on first-line treatment, 3.5 million on second-line treatment, and 0.6 million on third-line treatment). Our analysis showed that TAF and DTG will be major players in the ART regimen by 2025, with 8 million and 15 million patients using these ARVs respectively. However, as safety and efficacy of dolutegravir (DTG) and tenofovir alafenamide (TAF) during pregnancy and among TB/HIV co-infected patients using rifampicin is still under debate, and ART scale-up is predicted to increase considerably, there also remains a clear need for continuous supplies of existing ARVs including TDF and EFV, which 16 million and 10 million patients-respectively-are predicted to be using in 2025. It will be important to ensure that the existing capacities of generics manufacturers, which are geared towards ARVs of higher doses (such as TDF 300mg and EFV 600mg), will not be adversely impacted due to the introduction of lower dose ARVs such as TAF 25mg and DTG 50mg. With increased access to viral load testing, more patients would be using protease inhibitors containing regimens in second-line, with 1 million patients on LPV/r and 2.3 million on ATV/r by 2025. However, it will remain important to continue monitoring the evolution of ARV market in LMICs to guarantee the availability of these medicines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- UNAIDS. Fact Sheet 2016. Global Statistics 2015. Genva: UNAIDS; 2016. Available: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_e.... Accessed 6 August 2016).
-
- UNAIDS. How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva: UNAIDS; 2015. Available: http://www.unaids.org/en/resources/documents/2015/MDG6_15years-15lessons.... Accessed 6 August 2016.
-
- UNAIDS. FAST-TRACK: Ending the AIDS epidemic by 2030. Geneva: UNAIDS; 2014. Available:http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014repo.... Accessed 6 August 2016.
-
- UNAIDS. Understanding Fast Track: Accelerating action to end the AIDS epidemic by 2030. Geneva: UNAIDS; 2015. Available: http://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Unde..., Accessed 6 August 2016.
-
- ViiV Healthcare. Press Release. ViiV Healthcare announces U.S. approval of Tivicay® (dolutegravir) for the treatment of HIV-1. 12th August 2013. Available: https://www.viivhealthcare.com/media/press-releases/2013/august/viiv-hea.... Accessed 6 August 2016.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

