An RNAi-Based Control of Fusarium graminearum Infections Through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery
- PMID: 27737019
- PMCID: PMC5063301
- DOI: 10.1371/journal.ppat.1005901
An RNAi-Based Control of Fusarium graminearum Infections Through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery
Abstract
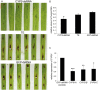
Meeting the increasing food and energy demands of a growing population will require the development of ground-breaking strategies that promote sustainable plant production. Host-induced gene silencing has shown great potential for controlling pest and diseases in crop plants. However, while delivery of inhibitory noncoding double-stranded (ds)RNA by transgenic expression is a promising concept, it requires the generation of transgenic crop plants which may cause substantial delay for application strategies depending on the transformability and genetic stability of the crop plant species. Using the agronomically important barley-Fusarium graminearum pathosystem, we alternatively demonstrate that a spray application of a long noncoding dsRNA (791 nt CYP3-dsRNA), which targets the three fungal cytochrome P450 lanosterol C-14α-demethylases, required for biosynthesis of fungal ergosterol, inhibits fungal growth in the directly sprayed (local) as well as the non-sprayed (distal) parts of detached leaves. Unexpectedly, efficient spray-induced control of fungal infections in the distal tissue involved passage of CYP3-dsRNA via the plant vascular system and processing into small interfering (si)RNAs by fungal DICER-LIKE 1 (FgDCL-1) after uptake by the pathogen. We discuss important consequences of this new finding on future RNA-based disease control strategies. Given the ease of design, high specificity, and applicability to diverse pathogens, the use of target-specific dsRNA as an anti-fungal agent offers unprecedented potential as a new plant protection strategy.
Conflict of interest statement
I have read the journal's policy and the fact that the authors VC, JM, and TM are employed by a commercial company BASF Resesarch Triangle Park and BASF Limburgerhof does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures


Comment in
-
Spray-Induced Gene Silencing: a Powerful Innovative Strategy for Crop Protection.Trends Microbiol. 2017 Jan;25(1):4-6. doi: 10.1016/j.tim.2016.11.011. Epub 2016 Dec 4. Trends Microbiol. 2017. PMID: 27923542 Free PMC article.
References
-
- FAO. FAO Statistical Yearbook. World Food and Agriculture. Part 3: Feeding the World (ISBN 978-92-5-107396-4). Accessed from Food and Agriculture Organization of the United Nations website: http://www.fao.org (2013)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

