Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial
- PMID: 27737436
- PMCID: PMC5824322
- DOI: 10.1001/jamaoncol.2016.3358
Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial
Erratum in
-
Incorrect Percentages in the Abstract.JAMA Oncol. 2017 Dec 1;3(12):1742. doi: 10.1001/jamaoncol.2017.4368. JAMA Oncol. 2017. PMID: 29145564 Free PMC article. No abstract available.
Abstract
Importance: Capecitabine is an oral cytotoxic chemotherapeutic commonly used across cancer subtypes. As with other oral medications though, it may suffer from drug interactions that could impair its absorption.
Objective: To determine if gastric acid suppressants such as proton pump inhibitors (PPIs) may impair capecitabine efficacy.
Design, setting, and participants: This secondary analysis of TRIO-013, a phase III randomized trial, compares capecitabine and oxaliplatin (CapeOx) with or without lapatinib in 545 patients with ERBB2/HER2-positive metastatic gastroesophageal cancer (GEC); patients were randomized 1:1 between CapeOx with or without lapatinib. Proton pump inhibitor use was identified by medication records. Progression-free survival (PFS) and overall survival (OS) were compared between patients treated with PPIs vs patients who were not. Specific subgroups were accounted for, such as younger age (<60 years), Asian ethnicity, female sex, and disease stage (metastatic/advanced) in multivariate Cox proportional hazards modeling. The TRIO-013 trial accrued and randomized patients between June 2008 and January 2012; this analysis took place in January 2014.
Interventions: Patients were divided based on PPI exposure.
Main outcomes and measures: Primary study outcome was PFS and OS between patients treated with PPIs vs patients who were not. Secondary outcomes included disease response rates and toxicities.
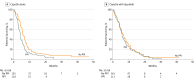
Results: Of the 545 patients with GEC (median age, 60 years; 406 men [74%]) included in the study, 229 received PPIs (42.0%) and were evenly distributed between arms. In the placebo arm, PPI-treated patients had poorer median PFS, 4.2 vs 5.7 months (hazard ratio [HR], 1.55; 95% CI, 1.29-1.81, P < .001); OS, 9.2 vs 11.3 months (HR, 1.34; 95% CI, 1.06-1.62; P = .04); and disease control rate (83% vs 72%; P = .02) vs patients not treated with PPIs. In multivariate analysis considering age, race, disease stage, and sex, PPI-treated patients had poorer PFS (HR, 1.68; 95% CI, 1.42-1.94; P < .001) and OS (HR, 1.41; 95% CI, 1.11-1.71; P = .001). In patients treated with CapeOx and lapatinib, PPIs had less effect on PFS (HR, 1.08; P = .54) and OS (HR, 1.26; P = .10); however, multivariate analysis in this group demonstrated a significant difference in OS (HR, 1.38; 95% CI, 1.06-1.66; P = .03).
Conclusions and relevance: Proton pump inhibitors negatively effected capecitabine efficacy by possibly raising gastric pH levels, leading to altered dissolution and absorption. These results are consistent with previous erlotinib and sunitinib studies. Whether PPIs affected lapatinib is unclear given concurrent capecitabine. Given capecitabine's prevalence in treatment breast cancer and colon cancer, further studies are under way.
Trial registration: clinicaltrials.gov Identifier: NCT00680901.
Conflict of interest statement
Figures

Comment in
-
Factors Affecting the Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer.JAMA Oncol. 2018 Feb 1;4(2):264. doi: 10.1001/jamaoncol.2017.3407. JAMA Oncol. 2018. PMID: 29098270 No abstract available.
-
Factors Affecting the Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer-Reply.JAMA Oncol. 2018 Feb 1;4(2):265. doi: 10.1001/jamaoncol.2017.3410. JAMA Oncol. 2018. PMID: 29098275 No abstract available.
-
Factors Affecting the Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer.JAMA Oncol. 2018 Feb 1;4(2):263. doi: 10.1001/jamaoncol.2016.6615. JAMA Oncol. 2018. PMID: 29098276 No abstract available.
-
Factors Affecting the Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer.JAMA Oncol. 2018 Feb 1;4(2):263-264. doi: 10.1001/jamaoncol.2016.7019. JAMA Oncol. 2018. PMID: 29098281 No abstract available.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. - PubMed
-
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523-1529. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. - PubMed
-
- Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362(9):858-859. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

