Effectiveness and cost-effectiveness of routine third trimester ultrasound screening for intrauterine growth restriction: study protocol of a nationwide stepped wedge cluster-randomized trial in The Netherlands (The IRIS Study)
- PMID: 27737654
- PMCID: PMC5064939
- DOI: 10.1186/s12884-016-1104-8
Effectiveness and cost-effectiveness of routine third trimester ultrasound screening for intrauterine growth restriction: study protocol of a nationwide stepped wedge cluster-randomized trial in The Netherlands (The IRIS Study)
Abstract
Background: Intrauterine growth retardation (IUGR) is a major risk factor for perinatal mortality and morbidity. Thus, there is a compelling need to introduce sensitive measures to detect IUGR fetuses. Routine third trimester ultrasonography is increasingly used to detect IUGR. However, we lack evidence for its clinical effectiveness and cost-effectiveness and information on ethical considerations of additional third trimester ultrasonography. This nationwide stepped wedge cluster-randomized trial examines the (cost-)effectiveness of routine third trimester ultrasonography in reducing severe adverse perinatal outcome through subsequent protocolized management.
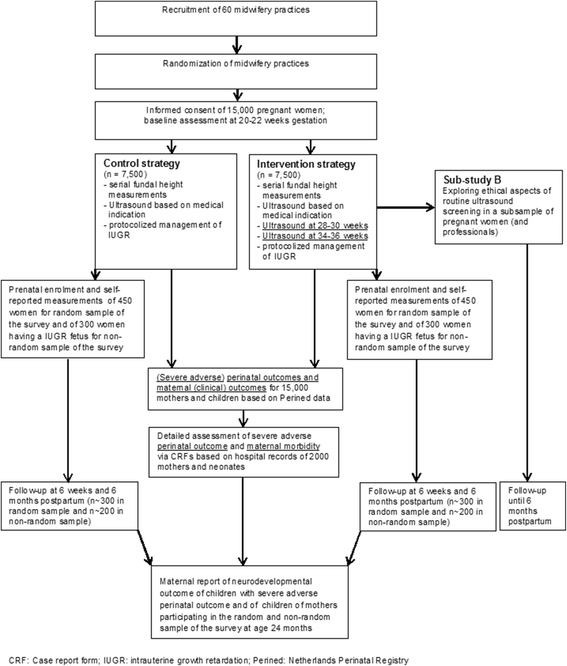
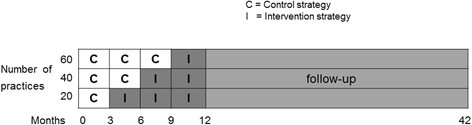
Methods: For this trial, 15,000 women with a singleton pregnancy receiving care in 60 participating primary care midwifery practices will be included at 22 weeks of gestation. In the intervention (n = 7,500) and control group (n = 7,500) fetal growth will be monitored by serial fundal height assessments. All practices will start offering the control condition (ultrasonography based on medical indication). Every three months, 20 practices will be randomized to the intervention condition, i.e. apart from ultrasonography if indicated, two routine ultrasound examinations will be performed (at 28-30 weeks and 34-36 weeks). If IUGR is suspected, both groups will receive subsequent clinical management as described in the IRIS study protocol that will be developed before the start of the trial. The primary dichotomous clinical composite outcome is 'severe adverse perinatal outcome' up to 7 days after birth, including: perinatal death; Apgar score <4 at 5 minutes after birth; impaired consciousness; need for assisted ventilation for more than 24 h; asphyxia; septicemia; meningitis; bronchopulmonary dysplasia; intraventricular hemorrhage; cystic periventricular leukomalacia; neonatal seizures or necrotizing enterocolitis. For the economic evaluation, costs will be measured from a societal perspective. Quality of life will be measured using the EQ-5D-5 L to enable calculation of QALYs. Cost-effectiveness and cost-utility analyses will be performed. In a qualitative sub-study (using diary notes from 32 women for 9 months, at least 10 individual interviews and 2 focus group studies) we will explore ethical considerations of additional ultrasonography and how to deal with them.
Discussion: The results of this trial will assist healthcare providers and policymakers in making an evidence-based decision about whether or not introducing routine third trimester ultrasonography.
Trial registration: NTR4367 , 21 March 2014.
Keywords: Intrauterine growth retardation; Midwifery; Perinatal outcome; Third trimester ultrasonography.
Figures
References
-
- Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013;208:290–296. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

