Effectiveness of a pharmacist-driven intervention in COPD (EPIC): study protocol for a randomized controlled trial
- PMID: 27737686
- PMCID: PMC5064938
- DOI: 10.1186/s13063-016-1623-7
Effectiveness of a pharmacist-driven intervention in COPD (EPIC): study protocol for a randomized controlled trial
Abstract
Background: Patients with chronic obstructive pulmonary disease (COPD) are often nonadherent with medications and have poor inhaler technique. Community pharmacists can help to improve health-related quality of life and overall outcomes in patients with COPD. We aim to measure the effectiveness of a systematic, pharmacist-driven intervention on patients with diagnosed COPD.
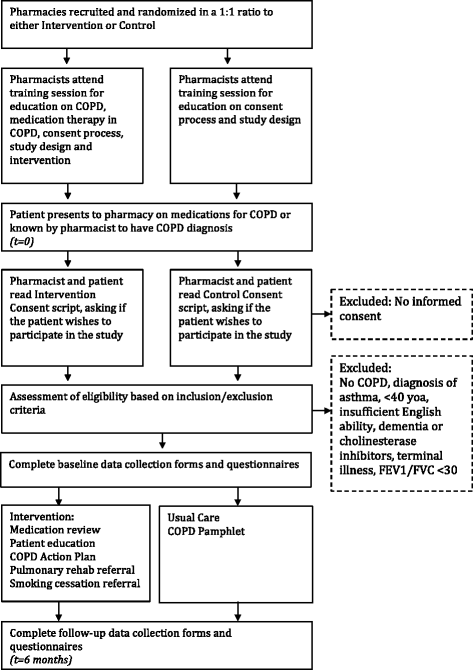
Methods/design: This pragmatic, parallel-group, cluster randomized controlled trial is designed to determine the effectiveness of a multifactorial, pharmacist-led intervention on medication adherence, inhaler technique, health-related quality of life, health care resource utilization including COPD exacerbations, and use of medications. Participating pharmacies in Newfoundland and Labrador (NL), Canada will be randomly assigned to either the intervention or the control group. The intervention group will deliver an enhanced form of care that emphasizes COPD management. The control group will provide usual care and a COPD education pamphlet. Included patients will be aged 40 years or older, have a physician-confirmed diagnosis of COPD, and be able to answer questionnaires in English. The primary outcomes are the between-group difference in the change from baseline to 6 months in medication adherence using the Medication Possession Ratio (MPR) and the Morisky Medication Adherence Scale (MMAS-8). The secondary outcomes are also measured from baseline to 6 months, and include the proportion of patients with a clinically significant change in adherence, the proportion of patients defined as having "good adherence," the mean MPR between groups, quality of life as measured by the St. George's Respiratory Questionnaire, medication inhalation technique using a pharmacist-scored checklist, health care resource utilization and antibiotic and orally administered corticosteroid use for COPD exacerbations. Differences between groups will be analyzed at the individual patient level while controlling for clustering effect.
Discussion: A pharmacist-led COPD intervention has the potential to improve patient medication adherence, thus increasing quality of life, possibly decreasing pulmonary exacerbations and reducing utilization of acute health care resources. Methods and results taken from this study could be used to enhance the delivery of COPD care by community pharmacists in a real-world setting. This would serve to enhance COPD population health and quality of life.
Trial registration: International Standard Randomized Controlled Trial Number (ISRCTN) ISRCTN78138190 , registered on 3 February 2016.
Keywords: Chronic obstructive pulmonary disease; Cluster randomized controlled trial; Community pharmacy; Medication adherence; Pharmacist; Pharmacy practice research.
References
-
- Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease;2015.
-
- Mathers C, Lopez A, Murray CJ. The burden of disease and mortality by condition: data, methods, and results for 2001. In: Lopez A, Mathers C, Ezzati M, Jamison D, Murray C, editors. Global burden of disease and risk factors, vol. Chapter 3. Washington (DC): World Bank; 2006. - PubMed
-
- Criner G, Bourbeau J, Diekemper R, Ouellette D, Goodridge D, Hernandez P, et al. Prevention of acute exacerbation of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894–942. doi: 10.1378/chest.14-1676. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical