Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission
- PMID: 27737949
- PMCID: PMC5204316
- DOI: 10.2337/db16-0915
Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission
Abstract
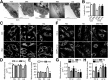
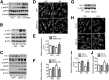
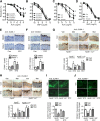
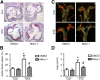
Metformin is a widely used antidiabetic drug that exerts cardiovascular protective effects in patients with diabetes. How metformin protects against diabetes-related cardiovascular diseases remains poorly understood. Here, we show that metformin abated the progression of diabetes-accelerated atherosclerosis by inhibiting mitochondrial fission in endothelial cells. Metformin treatments markedly reduced mitochondrial fragmentation, mitigated mitochondrial-derived superoxide release, improved endothelial-dependent vasodilation, inhibited vascular inflammation, and suppressed atherosclerotic lesions in streptozotocin (STZ)-induced diabetic ApoE-/- mice. In high glucose-exposed endothelial cells, metformin treatment and adenoviral overexpression of constitutively active AMPK downregulated mitochondrial superoxide, lowered levels of dynamin-related protein (Drp1) and its translocation into mitochondria, and prevented mitochondrial fragmentation. In contrast, AMPK-α2 deficiency abolished the effects of metformin on Drp1 expression, oxidative stress, and atherosclerosis in diabetic ApoE-/-/AMPK-α2-/- mice, indicating that metformin exerts an antiatherosclerotic action in vivo via the AMPK-mediated blockage of Drp1-mediated mitochondrial fission. Consistently, mitochondrial division inhibitor 1, a potent and selective Drp1 inhibitor, reduced mitochondrial fragmentation, attenuated oxidative stress, ameliorated endothelial dysfunction, inhibited inflammation, and suppressed atherosclerosis in diabetic mice. These findings show that metformin attenuated the development of atherosclerosis by reducing Drp1-mediated mitochondrial fission in an AMPK-dependent manner. Suppression of mitochondrial fission may be a therapeutic approach for treating macrovascular complications in patients with diabetes.
© 2017 by the American Diabetes Association.
Figures




References
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 - PubMed
-
- Mercer JR, Yu E, Figg N, et al. . The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/-/ApoE-/- mice. Free Radic Biol Med 2012;52:841–849 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

