Condensin II mutation causes T-cell lymphoma through tissue-specific genome instability
- PMID: 27737961
- PMCID: PMC5088566
- DOI: 10.1101/gad.284562.116
Condensin II mutation causes T-cell lymphoma through tissue-specific genome instability
Abstract
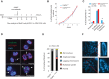
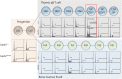
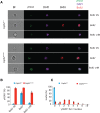
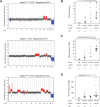
Chromosomal instability is a hallmark of cancer, but mitotic regulators are rarely mutated in tumors. Mutations in the condensin complexes, which restructure chromosomes to facilitate segregation during mitosis, are significantly enriched in cancer genomes, but experimental evidence implicating condensin dysfunction in tumorigenesis is lacking. We report that mice inheriting missense mutations in a condensin II subunit (Caph2nes) develop T-cell lymphoma. Before tumors develop, we found that the same Caph2 mutation impairs ploidy maintenance to a different extent in different hematopoietic cell types, with ploidy most severely perturbed at the CD4+CD8+ T-cell stage from which tumors initiate. Premalignant CD4+CD8+ T cells show persistent catenations during chromosome segregation, triggering DNA damage in diploid daughter cells and elevated ploidy. Genome sequencing revealed that Caph2 single-mutant tumors are near diploid but carry deletions spanning tumor suppressor genes, whereas P53 inactivation allowed Caph2 mutant cells with whole-chromosome gains and structural rearrangements to form highly aggressive disease. Together, our data challenge the view that mitotic chromosome formation is an invariant process during development and provide evidence that defective mitotic chromosome structure can promote tumorigenesis.
Keywords: chromosome structure; condensin; genome instability; lymphoma; mitosis.
© 2016 Woodward et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Brekelmans P, van Soest P, Voerman J, Platenburg PP, Leenen PJM, van Ewijk W. 1994. Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell Immunol 159: 331–339. - PubMed
-
- Bürmann F, Shin H-C, Basquin J, Soh Y-M, Giménez-Oya V, Kim Y-G, Oh B-H, Gruber S. 2013. An asymmetric SMC–kleisin bridge in prokaryotic condensin. Nat Struct Mol Biol 20: 371–379. - PubMed
-
- Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung M-W, Parsons JM, Steen MS, LaMadrid-Herrmannsfeldt MA, Williamson DW, Livingston RJ, et al. 2013. Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun 4: 2680. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
