Biliverdin Reductase A Attenuates Hepatic Steatosis by Inhibition of Glycogen Synthase Kinase (GSK) 3β Phosphorylation of Serine 73 of Peroxisome Proliferator-activated Receptor (PPAR) α
- PMID: 27738106
- PMCID: PMC5122784
- DOI: 10.1074/jbc.M116.731703
Biliverdin Reductase A Attenuates Hepatic Steatosis by Inhibition of Glycogen Synthase Kinase (GSK) 3β Phosphorylation of Serine 73 of Peroxisome Proliferator-activated Receptor (PPAR) α
Abstract
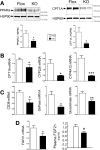
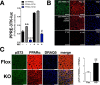
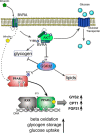
Non-alcoholic fatty liver disease is the most rapidly growing form of liver disease and if left untreated can result in non-alcoholic steatohepatitis, ultimately resulting in liver cirrhosis and failure. Biliverdin reductase A (BVRA) is a multifunctioning protein primarily responsible for the reduction of biliverdin to bilirubin. Also, BVRA functions as a kinase and transcription factor, regulating several cellular functions. We report here that liver BVRA protects against hepatic steatosis by inhibiting glycogen synthase kinase 3β (GSK3β) by enhancing serine 9 phosphorylation, which inhibits its activity. We show that GSK3β phosphorylates serine 73 (Ser(P)73) of the peroxisome proliferator-activated receptor α (PPARα), which in turn increased ubiquitination and protein turnover, as well as decreased activity. Interestingly, liver-specific BVRA KO mice had increased GSK3β activity and Ser(P)73 of PPARα, which resulted in decreased PPARα protein and activity. Furthermore, the liver-specific BVRA KO mice exhibited increased plasma glucose and insulin levels and decreased glycogen storage, which may be due to the manifestation of hepatic steatosis observed in the mice. These findings reveal a novel BVRA-GSKβ-PPARα axis that regulates hepatic lipid metabolism and may provide unique targets for the treatment of non-alcoholic fatty liver disease.
Keywords: glycogen synthase kinase 3 (GSK-3); heme oxygenase; liver metabolism; nuclear receptor; peroxisome proliferator-activated receptor (PPAR).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

