Oxalobacter formigenes- Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells
- PMID: 27738124
- PMCID: PMC5328155
- DOI: 10.1681/ASN.2016020132
Oxalobacter formigenes- Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells
Abstract
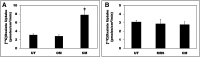
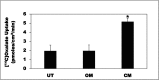
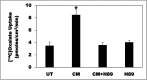
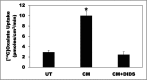
Hyperoxaluria is a major risk factor for kidney stones and has no specific therapy, although Oxalobacter formigenes colonization is associated with reduced stone risk. O. formigenes interacts with colonic epithelium and induces colonic oxalate secretion, thereby reducing urinary oxalate excretion, via an unknown secretagogue. The difficulties in sustaining O. formigenes colonization underscore the need to identify the derived factors inducing colonic oxalate secretion. We therefore evaluated the effects of O. formigenes culture conditioned medium (CM) on apical 14C-oxalate uptake by human intestinal Caco-2-BBE cells. Compared with control medium, O. formigenes CM significantly stimulated oxalate uptake (>2.4-fold), whereas CM from Lactobacillus acidophilus did not. Treating the O. formigenes CM with heat or pepsin completely abolished this bioactivity, and selective ultrafiltration of the CM revealed that the O. formigenes-derived factors have molecular masses of 10-30 kDa. Treatment with the protein kinase A inhibitor H89 or the anion exchange inhibitor 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid completely blocked the CM-induced oxalate transport. Knockdown of the oxalate transporter SLC26A6 also significantly restricted the induction of oxalate transport by CM. In a mouse model of primary hyperoxaluria type 1, rectal administration of O. formigenes CM significantly reduced (>32.5%) urinary oxalate excretion and stimulated (>42%) distal colonic oxalate secretion. We conclude that O. formigenes-derived bioactive factors stimulate oxalate transport in intestinal cells through mechanisms including PKA activation. The reduction in urinary oxalate excretion in hyperoxaluric mice treated with O. formigenes CM reflects the in vivo retention of biologic activity and the therapeutic potential of these factors.
Keywords: Oxalobacter formigenes; PKA; SLC26A6; intestinal oxalate transport; secreted bioactive factors.
Copyright © 2017 by the American Society of Nephrology.
Figures






References
-
- Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008 - PubMed
-
- Robertson WG, Peacock M: The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron 26: 105–110, 1980 - PubMed
-
- Kleinman JG: Bariatric surgery, hyperoxaluria, and nephrolithiasis: a plea for close postoperative management of risk factors. Kidney Int 72: 8–10, 2007 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

