Efficacy and Safety of Paliperidone Extended Release 1.5 mg/day-A Double-blind, Placebo- and Active-Controlled, Study in the Treatment of Patients with Schizophrenia
- PMID: 27738355
- PMCID: PMC5044480
- DOI: 10.64719/pb.4064
Efficacy and Safety of Paliperidone Extended Release 1.5 mg/day-A Double-blind, Placebo- and Active-Controlled, Study in the Treatment of Patients with Schizophrenia
Abstract
Background: Paliperidone extended-release (paliperidone ER) is an approved oral antipsychotic medication (dosing range 3-12 mg/day) for treatment of schizophrenia and schizoaffective disorder in adults.
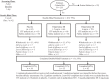
Methods: In this 3-arm, double-blind, placebo- and active-controlled, parallel-group study, paliperidone ER 1.5 mg was assessed to determine the lowest efficacious dose in patients (N = 201) with acute schizophrenia. Paliperidone ER 6 mg was included for assay sensitivity.
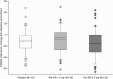
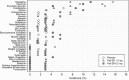
Results: Patients (intent-to-treat analysis set) had a mean age of 39.4 years; 74% were men, 43% Asian, and 40% black. The baseline mean (SD) Positive and Negative Syndrome Scale (PANSS) total score was 92.6 (13.02) and the mean (SD) change from baseline to endpoint was: placebo group, -11.4 (20.81); paliperidone ER 1.5 mg group, -8.9 (23.31); and paliperidone ER 6 mg group, -15.7 (26.25). Differences between paliperidone groups versus placebo were not significant (paliperidone ER 1.5 mg [p = 0.582], paliperidone ER 6 mg, [p = 0.308]). Safety results of paliperidone ER 1.5 mg and placebo were comparable. The most frequently reported treatment emergent adverse events (≥10%) were: placebo group-headache (15.6%) and psychotic disorder (14.1%); paliperidone ER 1.5 mg group-insomnia (13.6%); and paliperidone ER 6 mg group-headache (11.4%), insomnia (10%), and tremor (10%).
Conclusions: In this study, paliperidone ER 1.5 mg did not demonstrate efficacy in patients with acute schizophrenia. A markedly high placebo response was noted. Assay sensitivity with the 6 mg dose was not established. Paliperidone ER 1.5 mg was generally tolerable with a safety profile comparable to placebo.
Keywords: Antipsychotic; extended-release; lowest effective dose; paliperidone; schizophrenia.
Copyright © by MedWorks MediaGlobal, LLC., Los Angeles, CA All rights reserved. Printed in the United States.
Figures

References
-
- Lublin H, Eberhard J, Levander S. Current therapy issues and unmet clinical needs in the treatment of schizophrenia: a review of the new generation antipsychotics. Int Clin Psychopharmacol. 2005;20(4):183–198. - PubMed
-
- Invega® Prescribing Information. last update July 2009. Available at http://www.janssen.com/janssen/shared/pi/invega Accessed on 18 Jan, 2010.
-
- Davidson M, Emsley R, Kramer M, Ford L, Pan G, Lim P et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93(1-3):117–130. - PubMed
-
- Kane JM, Canas F, Kramer M, Ford L, Gassmann-Mayer C, Lim P et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1-3):147–161. - PubMed
-
- Marder SR, Kramer M, Ford L, Eerdekens E, Lim P, Eerdekens M et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–1370. - PubMed
LinkOut - more resources
Full Text Sources
