Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism
- PMID: 27739237
- PMCID: PMC6201314
- DOI: 10.1002/aur.1707
Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism
Abstract
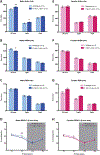
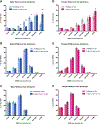
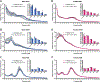
Sleep disturbances and hyperactivity are prevalent in several neurodevelopmental disorders, including autism spectrum disorders (ASDs) and attention deficit-hyperactivity disorder (ADHD). Evidence from genome-wide association studies indicates that chromosomal copy number variations (CNVs) are associated with increased prevalence of these neurodevelopmental disorders. In particular, CNVs in chromosomal region 16p11.2 profoundly increase the risk for ASD and ADHD, disorders that are more common in males than females. We hypothesized that mice hemizygous for the 16p11.2 deletion (16p11.2 del/+) would exhibit sex-specific sleep and activity alterations. To test this hypothesis, we recorded activity patterns using infrared beam breaks in the home-cage of adult male and female 16p11.2 del/+ and wildtype (WT) littermates. In comparison to controls, we found that both male and female 16p11.2 del/+ mice exhibited robust home-cage hyperactivity. In additional experiments, sleep was assessed by polysomnography over a 24-hr period. 16p11.2 del/+ male, but not female mice, exhibited significantly more time awake and significantly less time in non-rapid-eye-movement (NREM) sleep during the 24-hr period than wildtype littermates. Analysis of bouts of sleep and wakefulness revealed that 16p11.2 del/+ males, but not females, spent a significantly greater proportion of wake time in long bouts of consolidated wakefulness (greater than 42 min in duration) compared to controls. These changes in hyperactivity, wake time, and wake time distribution in the males resemble sleep disturbances observed in human ASD and ADHD patients, suggesting that the 16p11.2 del/+ mouse model may be a useful genetic model for studying sleep and activity problems in human neurodevelopmental disorders. Autism Res 2016. © 2016 International Society for Autism Research, Wiley Periodicals, Inc. Autism Res 2017, 10: 572-584. © 2016 International Society for Autism Research, Wiley Periodicals, Inc.
Keywords: 16p11.2 deletion; ADHD; autism; copy number variation; sex differences; sleep.
© 2016 International Society for Autism Research, Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Bazzett TJ, Becker JB (1994). Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Research, 637, 163–172. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

