Climate change and the ash dieback crisis
- PMID: 27739483
- PMCID: PMC5064362
- DOI: 10.1038/srep35303
Climate change and the ash dieback crisis
Abstract
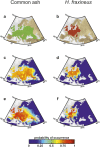
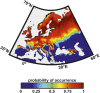
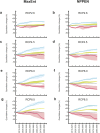
Beyond the direct influence of climate change on species distribution and phenology, indirect effects may also arise from perturbations in species interactions. Infectious diseases are strong biotic forces that can precipitate population declines and lead to biodiversity loss. It has been shown in forest ecosystems worldwide that at least 10% of trees are vulnerable to extinction and pathogens are increasingly implicated. In Europe, the emerging ash dieback disease caused by the fungus Hymenoscyphus fraxineus, commonly called Chalara fraxinea, is causing a severe mortality of common ash trees (Fraxinus excelsior); this is raising concerns for the persistence of this widespread tree, which is both a key component of forest ecosystems and economically important for timber production. Here, we show how the pathogen and climate change may interact to affect the future spatial distribution of the common ash. Using two presence-only models, seven General Circulation Models and four emission scenarios, we show that climate change, by affecting the host and the pathogen separately, may uncouple their spatial distribution to create a mismatch in species interaction and so a lowering of disease transmission. Consequently, as climate change expands the ranges of both species polewards it may alleviate the ash dieback crisis in southern and occidental regions at the same time.
Figures


References
-
- Harvell C. D. et al. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 (2002). - PubMed
-
- Kuussaari M. et al. Extinction debt: a challenge for biodiversity conservation. Trends Ecol Evol. 24, 564–571 (2009). - PubMed
-
- Altizer S., Ostfeld R. S., Johnson P. T. J., Kutz S. & Harvell C. D. Climate change and infectious diseases: From evidence to a predictive framework. Science 341, 514–519 (2013). - PubMed
-
- Smith M. D. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J Ecol. 99, 656–663 (2011).
-
- Allen C. D. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol Manag. 259, 660–684 (2010).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

