The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs)
- PMID: 27739509
- PMCID: PMC5064411
- DOI: 10.1038/srep35476
The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs)
Abstract
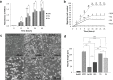
Recent studies have demonstrated that culture under hypoxia has beneficial effects on mesenchymal stem cells (MSCs). However, there are limitations to achieving a stable condition in conventional hypoxic CO2 incubators. DPSCs are a unique type of MSCs which are promising in many regenerative therapies. In this study, we investigated the ideal hypoxic culture environment for DPSCs using a new system that can provide controlled O2 environment. The effects of hypoxia (3%, 5%) on the stemness properties of DPSCs. Their morphology, proliferation rate, expression of stem cell markers, migration ability, mRNA expression of angiogenic/neurotrophic factors and immunomodulatory genes were evaluated and compared. Additionally, the effect of the discrete secretome on proliferation, migration, and neurogenic induction was assessed. Hypoxic DPSCs were found to be smaller in size and exhibited larger nuclei. 5% O2 significantly increased the proliferation rate, migration ability, expression of stem cell markers (CXCR4 and G-CSFR), and expression of SOX2, VEGF, NGF, and BDNF genes of DPSCs. Moreover, secretome collected from 5%O2 cultures displayed higher stimulatory effects on proliferation and migration of NIH3T3 cells and on neuronal differentiation of SH-SY5Y cells. These results demonstrate that 5%O2 may be ideal for enhancing DPSCs growth, stem cell properties, and secretome trophic effect.
Figures

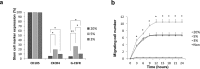
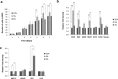

References
-
- Govindasamy V., Ronald V. S. & Abdullah A. N. Differentiation of dental pulp stem cells into islet-like aggregates. JDR. 90, 646–652 (2011). - PubMed
-
- Toma C., Pittenger M. F., Cahill K. S., Byrne B. J. & Kessler P. D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 105, 93–98 (2002). - PubMed
-
- Caplan A. I. & Dennis J. E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084 (2006). - PubMed
-
- Zhang M. et al.. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 21, 3197–3207 (2007). - PubMed
-
- Aggarwal S. & Pittenger M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105, 1815–1822 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

