Dynamic changes in the clonal structure of MDS and AML in response to epigenetic therapy
- PMID: 27740633
- PMCID: PMC5382101
- DOI: 10.1038/leu.2016.282
Dynamic changes in the clonal structure of MDS and AML in response to epigenetic therapy
Abstract
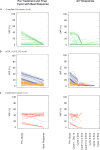
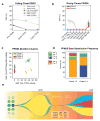
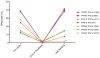
Traditional response criteria in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) are based on bone marrow morphology and may not accurately reflect clonal tumor burden in patients treated with non-cytotoxic chemotherapy. We used next-generation sequencing of serial bone marrow samples to monitor MDS and AML tumor burden during treatment with epigenetic therapy (decitabine and panobinostat). Serial bone marrow samples (and skin as a source of normal DNA) from 25 MDS and AML patients were sequenced (exome or 285 gene panel). We observed that responders, including those in complete remission (CR), can have persistent measurable tumor burden (that is, mutations) for at least 1 year without disease progression. Using an ultrasensitive sequencing approach, we detected extremely rare mutations (equivalent to 1 heterozygous mutant cell in 2000 non-mutant cells) months to years before their expansion at disease relapse. While patients can live with persistent clonal hematopoiesis in a CR or stable disease, ultimately we find evidence that expansion of a rare subclone occurs at relapse or progression. Here we demonstrate that sequencing of serial samples provides an alternative measure of tumor burden in MDS or AML patients and augments traditional response criteria that rely on bone marrow blast percentage.
Trial registration: ClinicalTrials.gov NCT00691938.
Conflict of interest statement
Conflict of interest statement: GLU has received compensation as a consultant for Novartis. The remaining authors report no relevant conflicts of interest.
Figures



References
-
- Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2009 Feb 1;28(4):556–561. - PubMed
-
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002 May 15;20(10):2429–2440. - PubMed
-
- Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006 Apr 15;106(8):1794–1803. - PubMed
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010 Feb 1;28(4):562–569. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

