Preclinical evaluation of a low-frequency transcranial MRI-guided focused ultrasound system in a primate model
- PMID: 27740941
- PMCID: PMC5079272
- DOI: 10.1088/0031-9155/61/21/7664
Preclinical evaluation of a low-frequency transcranial MRI-guided focused ultrasound system in a primate model
Abstract
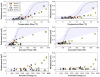
This study investigated thermal ablation and skull-induced heating with a 230 kHz transcranial MRI-guided focused ultrasound (TcMRgFUS) system in nonhuman primates. We evaluated real-time acoustic feedback and aimed to understand whether cavitation contributed to the heating and the lesion formation. In four macaques, we sonicated thalamic targets at acoustic powers of 34-560 W (896-7590 J). Tissue effects evaluated with MRI and histology were compared to MRI-based temperature and thermal dose measurements, acoustic emissions recorded during the experiments, and acoustic and thermal simulations. Peak temperatures ranged from 46 to 57 °C, and lesions were produced in 5/8 sonicated targets. A linear relationship was observed between the applied acoustic energy and both the focal and brain surface heating. Thermal dose thresholds were 15-50 cumulative equivalent minutes at 43 °C, similar to prior studies at higher frequencies. Histology was also consistent with earlier studies of thermal effects in the brain. The system successfully controlled the power level and maintained a low level of cavitation activity. Increased acoustic emissions observed in 3/4 animals occurred when the focal temperature rise exceeded approximately 16 °C. Thresholds for thermally-significant subharmonic and wideband emissions were 129 and 140 W, respectively, corresponding to estimated pressure amplitudes of 2.1 and 2.2 MPa. Simulated focal heating was consistent with the measurements for sonications without thermally-significant acoustic emissions; otherwise it was consistently lower than the measurements. Overall, these results suggest that the lesions were produced by thermal mechanisms. The detected acoustic emissions, however, and their association with heating suggest that cavitation might have contributed to the focal heating. Compared to earlier work with a 670 kHz TcMRgFUS system, the brain surface heating was substantially reduced and the focal heating was higher with this 230 kHz system, suggesting that a reduced frequency can increase the treatment envelope for TcMRgFUS and potentially reduce the risk of skull heating.
Figures


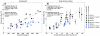







References
-
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858–61. - PubMed
-
- Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1):E1. - PubMed
-
- Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369(7):640–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
