Quality of pharmacoeconomic research in China: A systematic review
- PMID: 27741131
- PMCID: PMC5072958
- DOI: 10.1097/MD.0000000000005114
Quality of pharmacoeconomic research in China: A systematic review
Abstract
Background: The number of pharmacoeconomic publications in the literature from China has risen rapidly, but the quality of pharmacoeconomic publications from China has not been analyzed.
Objectives: This study aims to identify all recent pharmacoeconomic publications from China, to critically appraise the reporting quality, and to summarize the results.
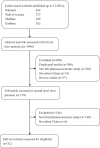
Methods: Four databases (PubMed, Web of Science, Medline, and EmBase) were searched for original articles published up to December 31, 2014. The Consolidated Health Economic Evaluation Reporting Standards statement including 24 items was used to assess the quality of reporting of these articles.
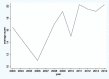
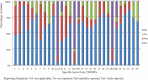
Results: Of 1046 articles identified, 32 studies fulfilled the inclusion criteria. They were published in 23 different journals. Quality of reporting varied between studies, with an average score of 18.7 (SD = 4.33) out of 24 (range 9-23.5). There was an increasing trend of pharmacoeconomic publications and reporting quality over years from 2003 to 2014. According to the Consolidated Health Economic Evaluation Reporting Standards, the reporting quality for the items including "title," "comparators of method," and "measurement of effectiveness" are quite low, with less than 50% of studies fully satisfying these reporting standards. In contrast, reporting was good for the items including "introduction," "study perspective," "choice of health outcomes," "study parameters," "characterizing heterogeneity," and "discussion," with more than 75% of the articles satisfying these reporting criteria. The remaining items fell in between these 2 extremes, with 50% to 75% of studies satisfying these criteria.
Conclusion: Our study suggests the need for improvement in a number of reporting criteria. But the criteria for which reporting quality was low seem to be limitations that would be straightforward to correct in future studies.
Conflict of interest statement
None of the authors have expressed any conflict of interest.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
A Systematic Review and Quality Assessment of Pharmacoeconomic Publications for China Compared to Internationally: Is the Quality of Evidence-Base Sufficient for Health Technology Assessment?Int J Health Policy Manag. 2025;14:8656. doi: 10.34172/ijhpm.8656. Epub 2025 Apr 28. Int J Health Policy Manag. 2025. PMID: 40767195 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
Cited by
-
Characteristics and Quality Appraisal of the Economic Evaluations Done in Ethiopia: A Systematic Review.Pharmacoecon Open. 2023 Nov;7(6):877-886. doi: 10.1007/s41669-023-00433-y. Epub 2023 Aug 25. Pharmacoecon Open. 2023. PMID: 37624553 Free PMC article.
-
Evaluation of Reporting Quality of Systematic Reviews on Health Economic Evaluation Studies Based on the CHEERS Statement: An Overview of Reviews.Iran J Public Health. 2024 Oct;53(10):2214-2225. doi: 10.18502/ijph.v53i10.16699. Iran J Public Health. 2024. PMID: 39544854 Free PMC article. Review.
-
Were economic evaluations well reported for the newly listed oncology drugs in China's national reimbursement drug list.BMC Health Serv Res. 2022 Dec 3;22(1):1475. doi: 10.1186/s12913-022-08858-7. BMC Health Serv Res. 2022. PMID: 36463141 Free PMC article.
-
Taking stock of cost-effectiveness analysis of healthcare in China.BMJ Glob Health. 2019 May 14;4(3):e001418. doi: 10.1136/bmjgh-2019-001418. eCollection 2019. BMJ Glob Health. 2019. PMID: 31179038 Free PMC article.
-
A retrospective cross-sectional descriptive study to critically appraise the quality of reporting of health economic evaluations conducted in the Indian setting.Perspect Clin Res. 2022 Jan-Mar;13(1):25-32. doi: 10.4103/picr.PICR_137_19. Epub 2021 Jan 8. Perspect Clin Res. 2022. PMID: 35198425 Free PMC article.
References
-
- Brown GC, Brown MM. Value-based medicine and pharmacoeconomics. Dev Ophthalmol 2016; 55:381–390. - PubMed
-
- Rodrigues J, Wu JH, Clay E, et al. Impact of pharmacoeconomics guidelines on the international publications in China. Value Health 2014; 17:A799. - PubMed
-
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed.New York: Oxford University Press; 2005.
-
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health care 2013; 29:117–122. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources