Acrolein preferentially damages nucleolus eliciting ribosomal stress and apoptosis in human cancer cells
- PMID: 27741518
- PMCID: PMC5348333
- DOI: 10.18632/oncotarget.12608
Acrolein preferentially damages nucleolus eliciting ribosomal stress and apoptosis in human cancer cells
Abstract
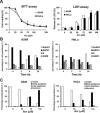
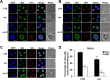
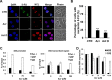
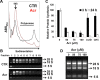
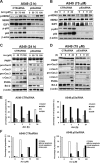
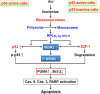
Acrolein (Acr) is a potent cytotoxic and DNA damaging agent which is ubiquitous in the environment and abundant in tobacco smoke. Acr is also an active cytotoxic metabolite of the anti-cancer drugs cyclophosphamide and ifosfamide. The mechanisms via which Acr exerts its anti-cancer activity and cytotoxicity are not clear. In this study, we found that Acr induces cytotoxicity and cell death in human cancer cells with different activities of p53. Acr preferentially binds nucleolar ribosomal DNA (rDNA) to form Acr-deoxyguanosine adducts, and induces oxidative damage to both rDNA and ribosomal RNA (rRNA). Acr triggers ribosomal stress responses, inhibits rRNA synthesis, reduces RNA polymerase I binding to the promoter of rRNA gene, disrupts nucleolar integrity, and impairs ribosome biogenesis and polysome formation. Acr causes an increase in MDM2 levels and phosphorylation of MDM2 in A549 and HeLa cells which are p53 active and p53 inactive, respectively. It enhances the binding of ribosomal protein RPL11 to MDM2 and reduces the binding of p53 and E2F-1 to MDM2 resulting in stabilization/activation of p53 in A549 cells and degradation of E2F-1 in A549 and HeLa cells. We propose that Acr induces ribosomal stress which leads to activation of MDM2 and RPL11-MDM2 binding, consequently, activates p53 and enhances E2F-1 degradation, and that taken together these two processes induce apoptosis and cell death.
Keywords: DNA damages; RPL11-MDM2-p53; acrolein; rDNA/ rRNA; ribosomal stress/ nucleolar stress.
Conflict of interest statement
No potential conflicts of interest were disclosed by the authors.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

