H2S-releasing nanoemulsions: a new formulation to inhibit tumor cells proliferation and improve tissue repair
- PMID: 27741519
- PMCID: PMC5356665
- DOI: 10.18632/oncotarget.12609
H2S-releasing nanoemulsions: a new formulation to inhibit tumor cells proliferation and improve tissue repair
Abstract
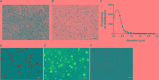
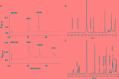
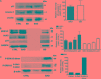
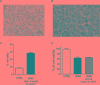
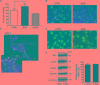
The improvement of solubility and/or dissolution rate of poorly soluble natural compounds is an ideal strategy to make them optimal candidates as new potential drugs. Accordingly, the allyl sulfur compounds and omega-3 fatty acids are natural hydrophobic compounds that exhibit two important combined properties: cardiovascular protection and antitumor activity. Here, we have synthesized and characterized a novel formulation of diallyl disulfide (DADS) and α-linolenic acid (ALA) as protein-nanoemulsions (BAD-NEs), using ultrasounds. BAD-NEs are stable over time at room temperature and show antioxidant and radical scavenging property. These NEs are also optimal H2S slow-release donors and show a significant anti-proliferative effect on different human cancer cell lines: MCF-7 breast cancer and HuT 78 T-cell lymphoma cells. BAD-NEs are able to regulate the ERK1/2 pathway, inducing apoptosis and cell cycle arrest at the G0/G1 phase. We have also investigated their effect on cell proliferation of human adult stem/progenitor cells. Interestingly, BAD-NEs are able to improve the Lin- Sca1+ human cardiac progenitor cells (hCPC) proliferation. This stem cell growth stimulation is combined with the expression and activation of proteins involved in tissue-repair, such as P-AKT, α-sma and connexin 43. Altogether, our results suggest that these antioxidant nanoemulsions might have potential application in selective cancer therapy and for promoting the muscle tissue repair.
Keywords: antioxidants; cancer; garlic; hydrogen sulfide; omega-3 fatty acid.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



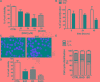
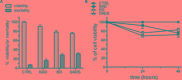

Similar articles
-
Molecular mechanism of diallyl disulfide in cell cycle arrest and apoptosis in HCT-116 colon cancer cells.J Biochem Mol Toxicol. 2009 Jan-Feb;23(1):71-9. doi: 10.1002/jbt.20266. J Biochem Mol Toxicol. 2009. PMID: 19202565
-
Effect of diallyl disulfide on insulin-like growth factor signaling molecules involved in cell survival and proliferation of human prostate cancer cells in vitro and in silico approach through docking analysis.Phytomedicine. 2012 Jul 15;19(10):912-23. doi: 10.1016/j.phymed.2012.04.009. Epub 2012 Jun 26. Phytomedicine. 2012. PMID: 22739413
-
Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the β-catenin signaling pathway.Mol Nutr Food Res. 2015 Jun;59(6):1063-75. doi: 10.1002/mnfr.201400668. Epub 2015 Apr 29. Mol Nutr Food Res. 2015. PMID: 25755089
-
Molecular mechanisms for the anti-cancer effects of diallyl disulfide.Food Chem Toxicol. 2013 Jul;57:362-70. doi: 10.1016/j.fct.2013.04.001. Epub 2013 Apr 9. Food Chem Toxicol. 2013. PMID: 23583486 Review.
-
Anticancer effects of garlic and garlic-derived compounds for breast cancer control.Anticancer Agents Med Chem. 2011 Mar;11(3):249-53. doi: 10.2174/187152011795347441. Anticancer Agents Med Chem. 2011. PMID: 21269259 Review.
Cited by
-
Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress.Int J Mol Sci. 2018 Aug 11;19(8):2368. doi: 10.3390/ijms19082368. Int J Mol Sci. 2018. PMID: 30103516 Free PMC article.
-
New immunological potential markers for triple negative breast cancer: IL18R1, CD53, TRIM, Jaw1, LTB, PTPRCAP.Discov Oncol. 2021 Mar 10;12(1):6. doi: 10.1007/s12672-021-00401-0. Discov Oncol. 2021. PMID: 35201443 Free PMC article.
-
Global mapping of cancers: The Cancer Genome Atlas and beyond.Mol Oncol. 2021 Nov;15(11):2823-2840. doi: 10.1002/1878-0261.13056. Epub 2021 Jul 20. Mol Oncol. 2021. PMID: 34245122 Free PMC article. Review.
-
Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3D-Cell Culture Systems Optimization.Int J Mol Sci. 2021 Jun 5;22(11):6095. doi: 10.3390/ijms22116095. Int J Mol Sci. 2021. PMID: 34198821 Free PMC article.
-
Micronutrient Status and Breast Cancer: A Narrative Review.Int J Mol Sci. 2024 May 2;25(9):4968. doi: 10.3390/ijms25094968. Int J Mol Sci. 2024. PMID: 38732186 Free PMC article. Review.
References
-
- Dirsch VM, Gerbes AL, Vollmar AM. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaB. Mol Pharmacol. 1998;53:402–7. - PubMed
-
- Knowles LM, Milner JA. Allyl sulfides modify cell growth. Drug Metabol Drug Interact. 2000;17:81–107. - PubMed
-
- Lea MA. Organosulfur compounds and cancer. Adv Exp Med Biol. 1996;401:147–54. - PubMed
-
- Lea MA, Randolph VM, Patel M. Increased acetylation of histones induced by diallyl disulfide and structurally related molecules. Int J Oncol. 1999;15:347–52. - PubMed
-
- Li G, Qiao C, Lin R, Pinto J, Osborne M, Tiwari R. Antiproliferative effects of garlic constituents in cultured human breast-cancer cells. Oncol Rep. 1995;2:787–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

