DNA Polymerase ν Rapidly Bypasses O6-Methyl-dG but Not O6-[4-(3-Pyridyl)-4-oxobutyl-dG and O2-Alkyl-dTs
- PMID: 27741574
- PMCID: PMC5673091
- DOI: 10.1021/acs.chemrestox.6b00318
DNA Polymerase ν Rapidly Bypasses O6-Methyl-dG but Not O6-[4-(3-Pyridyl)-4-oxobutyl-dG and O2-Alkyl-dTs
Abstract
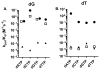
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent tobacco carcinogen that forms mutagenic DNA adducts including O6-methyl-2'-deoxyguanosine (O6-Me-dG), O6-[4-(3-pyridyl)-4-oxobut-1-yl]-dG (O6-POB-dG), O2-methylthymidine (O2-Me-dT), and O2-POB-dT. We evaluated the ability of human DNA polymerase ν to bypass this damage to evaluate the structural constraints on substrates for pol ν and to evaluate if there is kinetic evidence suggesting the in vivo activity of pol ν on tobacco-induced DNA damage. Presteady-state kinetic analysis has indicated that O6-Me-dG is a good substrate for pol ν, while O6-POB-dG and the O2-alkyl-dT adducts are poor substrates for pol ν. The reactivity with O6-Me-dG is high with a preference for dCTP > dGTP > dATP > dTTP. The catalytic activity of pol ν toward O6-Me-dG is high and can potentially be involved in its bypass in vivo. In contrast, pol ν is unlikely to bypass O6-POB-dG or the O2-alkyl-dTs in vivo.
Conflict of interest statement
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. The authors declare no competing financial interest.
Figures
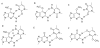



Similar articles
-
The roles of polymerases ν and θ in replicative bypass of O6- and N2-alkyl-2'-deoxyguanosine lesions in human cells.J Biol Chem. 2020 Apr 3;295(14):4556-4562. doi: 10.1074/jbc.RA120.012830. Epub 2020 Feb 25. J Biol Chem. 2020. PMID: 32098870 Free PMC article.
-
DNA Polymerases η and ζ Combine to Bypass O(2)-[4-(3-Pyridyl)-4-oxobutyl]thymine, a DNA Adduct Formed from Tobacco Carcinogens.Chem Res Toxicol. 2016 Mar 21;29(3):303-16. doi: 10.1021/acs.chemrestox.5b00468. Epub 2016 Feb 22. Chem Res Toxicol. 2016. PMID: 26868090 Free PMC article.
-
Roles of translesion synthesis DNA polymerases in the potent mutagenicity of tobacco-specific nitrosamine-derived O2-alkylthymidines in human cells.DNA Repair (Amst). 2015 Nov;35:63-70. doi: 10.1016/j.dnarep.2015.09.023. Epub 2015 Sep 21. DNA Repair (Amst). 2015. PMID: 26460881 Free PMC article.
-
Low fidelity bypass of O(2)-(3-pyridyl)-4-oxobutylthymine, the most persistent bulky adduct produced by the tobacco specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by model DNA polymerases.Chem Res Toxicol. 2012 Jun 18;25(6):1195-202. doi: 10.1021/tx200483g. Epub 2012 May 1. Chem Res Toxicol. 2012. PMID: 22533615
-
Kinetics of O(6)-pyridyloxobutyl-2'-deoxyguanosine repair by human O(6)-alkylguanine DNA alkyltransferase.Biochemistry. 2013 Jun 11;52(23):4075-88. doi: 10.1021/bi4004952. Epub 2013 May 31. Biochemistry. 2013. PMID: 23683164 Free PMC article.
Cited by
-
Beyond the Lesion: Back to High Fidelity DNA Synthesis.Front Mol Biosci. 2022 Jan 5;8:811540. doi: 10.3389/fmolb.2021.811540. eCollection 2021. Front Mol Biosci. 2022. PMID: 35071328 Free PMC article. Review.
-
The roles of polymerases ν and θ in replicative bypass of O6- and N2-alkyl-2'-deoxyguanosine lesions in human cells.J Biol Chem. 2020 Apr 3;295(14):4556-4562. doi: 10.1074/jbc.RA120.012830. Epub 2020 Feb 25. J Biol Chem. 2020. PMID: 32098870 Free PMC article.
-
Computational insights into the mutagenicity of two tobacco-derived carcinogenic DNA lesions.Nucleic Acids Res. 2018 Dec 14;46(22):11858-11868. doi: 10.1093/nar/gky1071. Nucleic Acids Res. 2018. PMID: 30407571 Free PMC article.
References
-
- Cancer Facts and Figures, 2014. American Cancer Society; Atlanta, GA: 2014.
-
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. - PubMed
-
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. - PubMed
-
- Hecht SS, Jordan KG, Choi CI, Trushin N. Effects of deuterium substitution on the tumorigenicity of 4-(metlhylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosa-mino)-1-(3-pyridyl)-1-butanol in A/J mice. Carcinogenesis. 1990;11:1017–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

