Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial
- PMID: 27741949
- PMCID: PMC5065699
- DOI: 10.1186/s13054-016-1511-2
Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial
Abstract
Background: Authors of recent meta-analyses have reported that prolonged glucocorticoid treatment is associated with significant improvements in patients with severe pneumonia or acute respiratory distress syndrome (ARDS) of multifactorial etiology. A prospective randomized trial limited to patients with sepsis-associated ARDS is lacking. The objective of our study was to evaluate the efficacy of hydrocortisone treatment in sepsis-associated ARDS.
Methods: In this double-blind, single-center (Siriraj Hospital, Bangkok), randomized, placebo-controlled trial, we recruited adult patients with severe sepsis within 12 h of their meeting ARDS criteria. Patients were randomly assigned (1:1 ratio) to receive either hydrocortisone 50 mg every 6 h or placebo. The primary endpoint was 28-day all-cause mortality; secondary endpoints included survival without organ support on day 28.
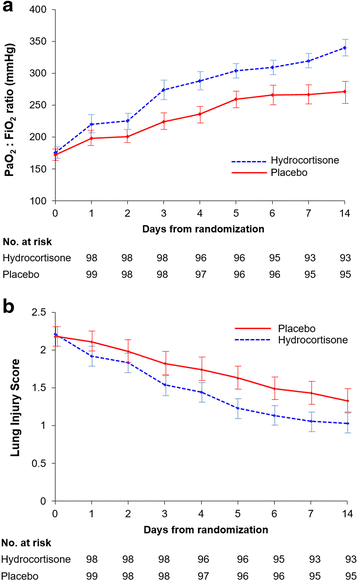
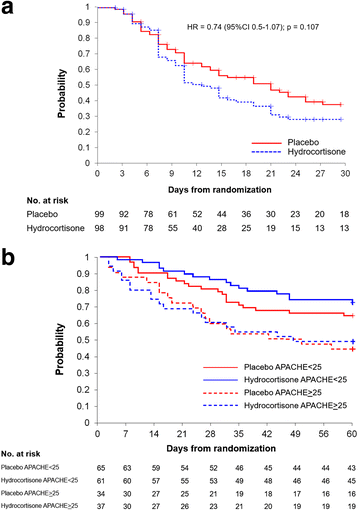
Results: Over the course of 4 years, 197 patients were randomized to either hydrocortisone (n = 98) or placebo (n = 99) and were included in this intention-to-treat analysis. The treatment group had significant improvement in the ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen and lung injury score (p = 0.01), and similar timing to removal of vital organ support (HR 0.74, 95 % CI 0.51-1.07; p = 0.107). After adjustment for significant covariates, day 28 survival was similar for the whole group (HR 0.80, 95 % CI 0.46-1.41; p = 0.44) and for the larger subgroup (n = 126) with Acute Physiology and Chronic Health Evaluation II score <25 (HR 0.57, 95 % CI 0.24-1.36; p = 0.20). With the exception of hyperglycemia (80.6 % vs. 67.7 %; p = 0.04), the rate of adverse events was similar. Hyperglycemia had no impact on outcome.
Conclusions: In sepsis-associated ARDS, hydrocortisone treatment was associated with a significant improvement in pulmonary physiology, but without a significant survival benefit.
Trial registration: ClinicalTrials.gov identifier NCT01284452 . Registered on 18 January 2011.
Keywords: ARDS; Glucocorticoid treatment; Hydrocortisone; Randomized trial; Septic shock; Severe sepsis.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

