Making a membrane on the other side of the wall
- PMID: 27742351
- PMCID: PMC5388599
- DOI: 10.1016/j.bbalip.2016.10.004
Making a membrane on the other side of the wall
Abstract
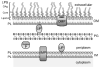
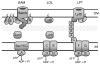
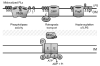
The outer membrane (OM) of Gram-negative bacteria is positioned at the frontline of the cell's interaction with its environment and provides a barrier against influx of external toxins while still allowing import of nutrients and excretion of wastes. It is a remarkable asymmetric bilayer with a glycolipid surface-exposed leaflet and a glycerophospholipid inner leaflet. Lipid asymmetry is key to OM barrier function and several different systems actively maintain this lipid asymmetry. All OM components are synthesized in the cytosol before being secreted and assembled into a contiguous membrane on the other side of the cell wall. Work in recent years has uncovered the pathways that transport and assemble most of the OM components. However, our understanding of how phospholipids are delivered to the OM remains notably limited. Here we will review seminal works in phospholipid transfer performed some 40years ago and place more recent insights in their context. This article is part of a Special Issue entitled: Bacterial Lipids edited by Russell E. Bishop.
Keywords: Gram-negative bacteria; Lipopolysaccharide; Lipoproteins; Outer membrane; Phospholipids; β-barrel proteins.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures

References
-
- Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976;15:2561–2570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

