Claudin-1 promotes TNF-α-induced epithelial-mesenchymal transition and migration in colorectal adenocarcinoma cells
- PMID: 27742576
- PMCID: PMC6166648
- DOI: 10.1016/j.yexcr.2016.10.005
Claudin-1 promotes TNF-α-induced epithelial-mesenchymal transition and migration in colorectal adenocarcinoma cells
Abstract
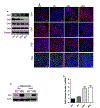
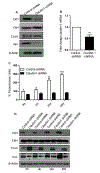
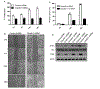
Epithelial-mesenchymal transition (EMT) is an important mechanism in cancer progression and malignancy including colorectal cancer (CRC). Importantly, inflammatory mediators are critical constituents of the local tumor environment and an intimate link between CRC progression and inflammation is now validated. We and others have reported key role of the deregulated claudin-1 expression in colon carcinogenesis including colitis-associated colon cancer (CAC). However, the causal association between claudin-1 expression and inflammation-induced colon cancer progression remains unclear. Here we demonstrate, TNF-α, a pro-inflammatory cytokine, regulates claudin-1 to modulate epithelial to mesenchymal transition (EMT) and migration in colon adenocarcinoma cells. Importantly, colon cancer cells cultured in the presence of TNF-α (10ng/ml), demonstrated a sharp decrease in E-cadherin expression and an increase in vimentin expression (versus control cells). Interestingly, TNF-α treatment also upregulated (and delocalized) claudin-1 expression in a time-dependent manner accompanied by increase in proliferation and wound healing. Furthermore, similar to our previous observation that claudin-1 overexpression in CRC cells induces ERK1/2 and Src- activation, signaling associated with colon cancer cell survival and transformation, TNF-α-treatment induced upregulation of phospho-ERK1/2 and -Src expression. The shRNA-mediated inhibition of claudin-1 expression largely abrogated the TNF-α-induced changes in EMT, proliferation, migration, p-Erk and p-Src expression. Taken together, our data demonstrate TNF-α mediated regulation of claudin-1 and tumorigenic abilities of colon cancer cells and highlights a key role of deregulated claudin-1 expression in inflammation-induced colorectal cancer growth and progression, through the regulation of the ERK and Src-signaling.
Keywords: Claudin-1; EMT; Migration; TNF-alpha.
Published by Elsevier Inc.
Figures


References
-
- Kinugasa T, Akagi Y, Yoshida T, Ryu Y, Shiratuchi I, Ishibashi N, et al., Increased claudin-1 protein expression contributes to tumorigenesis in ulcerative colitis-associated colorectal cancer, Anticancer Res 30 (8) (2010) 3181–3186. - PubMed
-
- Nakagawa S, Miyoshi N, Ishii H, Mimori K, Tanaka F, Sekimoto M, et al., Expression of CLDN1 in colorectal cancer: a novel marker for prognosis, Int. J. Oncol 39 (4) (2011) 791–796. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

