Cell-Free Synthetic Biology: Engineering Beyond the Cell
- PMID: 27742731
- PMCID: PMC5131772
- DOI: 10.1101/cshperspect.a023853
Cell-Free Synthetic Biology: Engineering Beyond the Cell
Abstract
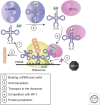
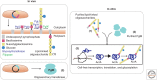
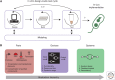
Cell-free protein synthesis (CFPS) technologies have enabled inexpensive and rapid recombinant protein expression. Numerous highly active CFPS platforms are now available and have recently been used for synthetic biology applications. In this review, we focus on the ability of CFPS to expand our understanding of biological systems and its applications in the synthetic biology field. First, we outline a variety of CFPS platforms that provide alternative and complementary methods for expressing proteins from different organisms, compared with in vivo approaches. Next, we review the types of proteins, protein complexes, and protein modifications that have been achieved using CFPS systems. Finally, we introduce recent work on genetic networks in cell-free systems and the use of cell-free systems for rapid prototyping of in vivo networks. Given the flexibility of cell-free systems, CFPS holds promise to be a powerful tool for synthetic biology as well as a protein production technology in years to come.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Albayrak C, Swartz JR. 2013b. Using E. coli-based cell-free protein synthesis to evaluate the kinetic performance of an orthogonal tRNA and aminoacyl-tRNA synthetase pair. Biochem Biophys Res Commun 431: 291–295. - PubMed
-
- Alfonta L, Zhang Z, Uryu S, Loo JA, Schultz PG. 2003. Site-specific incorporation of a redox-active amino acid into proteins. J Am Chem Soc 125: 14662–14663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
