β-Lactamases: A Focus on Current Challenges
- PMID: 27742735
- PMCID: PMC5204326
- DOI: 10.1101/cshperspect.a025239
β-Lactamases: A Focus on Current Challenges
Abstract
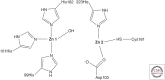
β-Lactamases, the enzymes that hydrolyze β-lactam antibiotics, remain the greatest threat to the usage of these agents. In this review, the mechanism of hydrolysis is discussed for both those enzymes that use serine at the active site and those that require divalent zinc ions for hydrolysis. The β-lactamases now include >2000 unique, naturally occurring amino acid sequences. Some of the clinically most important of these are the class A penicillinases, the extended-spectrum β-lactamases (ESBLs), the AmpC cephalosporinases, and the carbapenem-hydrolyzing enzymes in both the serine and metalloenzyme groups. Because of the versatility of these enzymes to evolve as new β-lactams are used therapeutically, new approaches to antimicrobial therapy may be required.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Abraham EP, Chain E. 1988. An enzyme from bacteria able to destroy penicillin. Rev Infect Dis 10: 677–678. - PubMed
-
- Bauvois C, Wouters J. 2007. Crystal structures of class C β-lactamases: Mechanistic implications and perspectives in drug design. In Enzyme-mediated resistance to antibiotics: Mechanisms, dissemination, and prospects for inhibition (ed. Bonomo RA, Tolmasky ME), pp. 145–161. ASM, Washington DC.
-
- Bush K. 2010a. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol 13: 558–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical