Neural tube morphogenesis in synthetic 3D microenvironments
- PMID: 27742791
- PMCID: PMC5098636
- DOI: 10.1073/pnas.1603529113
Neural tube morphogenesis in synthetic 3D microenvironments
Erratum in
-
Correction for Ranga et al., Neural tube morphogenesis in synthetic 3D microenvironments.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):E3163. doi: 10.1073/pnas.1703993114. Proc Natl Acad Sci U S A. 2017. PMID: 28396422 Free PMC article. No abstract available.
Abstract
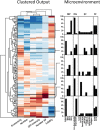
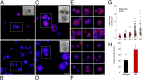
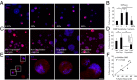
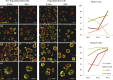
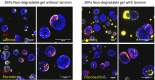
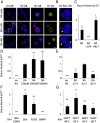
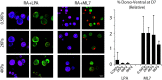
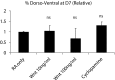
Three-dimensional organoid constructs serve as increasingly widespread in vitro models for development and disease modeling. Current approaches to recreate morphogenetic processes in vitro rely on poorly controllable and ill-defined matrices, thereby largely overlooking the contribution of biochemical and biophysical extracellular matrix (ECM) factors in promoting multicellular growth and reorganization. Here, we show how defined synthetic matrices can be used to explore the role of the ECM in the development of complex 3D neuroepithelial cysts that recapitulate key steps in early neurogenesis. We demonstrate how key ECM parameters are involved in specifying cytoskeleton-mediated symmetry-breaking events that ultimately lead to neural tube-like patterning along the dorsal-ventral (DV) axis. Such synthetic materials serve as valuable tools for studying the discrete action of extrinsic factors in organogenesis, and allow for the discovery of relationships between cytoskeletal mechanobiology and morphogenesis.
Keywords: development; embryonic stem cell; extracellular matrix; neural tube; organoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kojima Y, Tam OH, Tam PP. Timing of developmental events in the early mouse embryo. Semin Cell Dev Biol. 2014;34:65–75. - PubMed
-
- Stern CD, Fraser SE. Tracing the lineage of tracing cell lineages. Nat Cell Biol. 2001;3(9):E216–E218. - PubMed
-
- Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

