Modulating the Effects of the Bacterial Chaperonin GroEL on Fibrillogenic Polypeptides through Modification of Domain Hinge Architecture
- PMID: 27742838
- PMCID: PMC5122787
- DOI: 10.1074/jbc.M116.751925
Modulating the Effects of the Bacterial Chaperonin GroEL on Fibrillogenic Polypeptides through Modification of Domain Hinge Architecture
Abstract
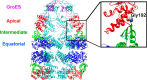
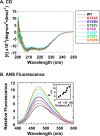
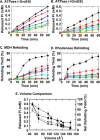
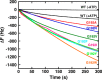
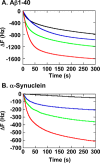
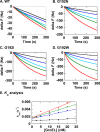
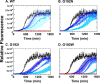
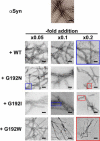
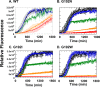
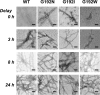
The isolated apical domain of the Escherichia coli GroEL subunit displays the ability to suppress the irreversible fibrillation of numerous amyloid-forming polypeptides. In previous experiments, we have shown that mutating Gly-192 (located at hinge II that connects the apical domain and the intermediate domain) to a tryptophan results in an inactive chaperonin whose apical domain is disoriented. In this study, we have utilized this disruptive effect of Gly-192 mutation to our advantage, by substituting this residue with amino acid residues of varying van der Waals volumes with the intent to modulate the affinity of GroEL toward fibrillogenic peptides. The affinities of GroEL toward fibrillogenic polypeptides such as Aβ(1-40) (amyloid-β(1-40)) peptide and α-synuclein increased in accordance to the larger van der Waals volume of the substituent amino acid side chain in the G192X mutants. When we compared the effects of wild-type GroEL and selected GroEL G192X mutants on α-synuclein fibril formation, we found that the effects of the chaperonin on α-synuclein fibrillation were different; the wild-type chaperonin caused changes in both the initial lag phase and the rate of fibril extension, whereas the effects of the G192X mutants were more specific toward the nucleus-forming lag phase. The chaperonins also displayed differential effects on α-synuclein fibril morphology, suggesting that through mutation of Gly-192, we may induce changes to the intermolecular affinities between GroEL and α-synuclein, leading to more efficient fibril suppression, and in specific cases, modulation of fibril morphology.
Keywords: GroEL; alpha-synuclein (a-synuclein); amyloid fibrils; chaperonin; protein aggregation; protein engineering; protein misfolding; protein-protein interaction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Hayer-Hartl M., Bracher A., and Hartl F. U. (2016) The GroEL-GroES chaperonin machine: a nano-cage for protein folding. Trends Biochem. Sci. 41, 62–76 - PubMed
-
- Saibil H. R., Fenton W. A., Clare D. K., and Horwich A. L. (2013) Structure and allostery of the chaperonin GroEL. J. Mol. Biol. 425, 1476–1487 - PubMed
-
- Sharma S., Chakraborty K., Müller B. K., Astola N., Tang Y. C., Lamb D. C., Hayer-Hartl M., and Hartl F. U. (2008) Monitoring protein conformation along the pathway of chaperonin-assisted folding. Cell 133, 142–153 - PubMed
-
- Chaudhuri T. K., Verma V. K., and Maheshwari A. (2009) GroEL assisted folding of large polypeptide substrates in Escherichia coli: present scenario and assignments for the future. Prog. Biophys. Mol. Biol. 99, 42–50 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

