Dopaminergic inhibition of gonadotropin-releasing hormone neurons in the cichlid fish Astatotilapia burtoni
- PMID: 27742893
- PMCID: PMC5201004
- DOI: 10.1242/jeb.147637
Dopaminergic inhibition of gonadotropin-releasing hormone neurons in the cichlid fish Astatotilapia burtoni
Abstract
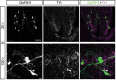
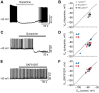
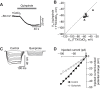
Dopamine regulates reproduction in part by modulating neuronal activity within the hypothalamic-pituitary-gonadal (HPG) axis. Previous studies suggested numerous mechanisms by which dopamine exerts inhibitory control over the HPG axis, ultimately changing the levels of sex steroids that regulate reproductive behaviors. However, it is not known whether these mechanisms are conserved across vertebrate species. In particular, it is unknown whether mechanisms underlying dopaminergic control of reproduction are shared between mammals and teleost fish. In mammals, dopamine directly inhibits gonadotropin-releasing hormone (GnRH1) hypothalamic neurons, the gatekeepers for activation of the HPG axis. Here, we demonstrate, for the first time in teleost fish, dopaminergic control of GnRH1 neurons via direct dopamine type-2-like receptor (D2R)-mediated inhibition within the hypothalamus. These results suggest that direct dopaminergic control of GnRH1 neurons via interactions in the hypothalamus is not exclusive to tetrapod reproductive control, but is likely conserved across vertebrate species.
Keywords: Dopamine; GnRH; HPG axis; Hypothalamus; Reproduction.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
Electrical synapses connect a network of gonadotropin releasing hormone neurons in a cichlid fish.Proc Natl Acad Sci U S A. 2015 Mar 24;112(12):3805-10. doi: 10.1073/pnas.1421851112. Epub 2015 Mar 9. Proc Natl Acad Sci U S A. 2015. PMID: 25775522 Free PMC article.
-
Social regulation of the electrical properties of gonadotropin-releasing hormone neurons in a cichlid fish (Astatotilapia burtoni).Biol Reprod. 2004 Sep;71(3):909-18. doi: 10.1095/biolreprod.104.030072. Epub 2004 May 12. Biol Reprod. 2004. PMID: 15140799
-
Androgen receptors in a cichlid fish, Astatotilapia burtoni: structure, localization, and expression levels.J Comp Neurol. 2007 Sep 1;504(1):57-73. doi: 10.1002/cne.21435. J Comp Neurol. 2007. PMID: 17614300 Free PMC article.
-
Neuroendocrinology of reproduction in teleost fish.Gen Comp Endocrinol. 2010 Feb 1;165(3):438-55. doi: 10.1016/j.ygcen.2009.04.017. Epub 2009 Apr 23. Gen Comp Endocrinol. 2010. PMID: 19393655 Review.
-
Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function.Front Neuroendocrinol. 2010 Jul;31(3):284-95. doi: 10.1016/j.yfrne.2010.03.001. Epub 2010 Mar 6. Front Neuroendocrinol. 2010. PMID: 20211640 Review.
Cited by
-
Sex- and brain region-specific patterns of gene expression associated with socially-mediated puberty in a eusocial mammal.PLoS One. 2018 Feb 23;13(2):e0193417. doi: 10.1371/journal.pone.0193417. eCollection 2018. PLoS One. 2018. PMID: 29474488 Free PMC article.
-
Mechanistic target of rapamycin (mTOR) implicated in plasticity of the reproductive axis during social status transitions.Gen Comp Endocrinol. 2019 Oct 1;282:113209. doi: 10.1016/j.ygcen.2019.113209. Epub 2019 Jun 18. Gen Comp Endocrinol. 2019. PMID: 31226256 Free PMC article.
-
Organization of the Catecholaminergic System in the Short-Lived Fish Nothobranchius furzeri.Front Neuroanat. 2021 Sep 13;15:728720. doi: 10.3389/fnana.2021.728720. eCollection 2021. Front Neuroanat. 2021. PMID: 34588961 Free PMC article.
-
Histological and transcriptomic effects of 17α-methyltestosterone on zebrafish gonad development.BMC Genomics. 2017 Jul 24;18(1):557. doi: 10.1186/s12864-017-3915-z. BMC Genomics. 2017. PMID: 28738802 Free PMC article.
-
The Dopamine D4 Receptor Regulates Gonadotropin-Releasing Hormone Neuron Excitability in Male Mice.eNeuro. 2022 Mar 3;9(2):ENEURO.0461-21.2022. doi: 10.1523/ENEURO.0461-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35165199 Free PMC article.
References
-
- Contijoch A. M., Gonzalez C., Singh H. N., Malamed S., Troncoso S. and Advis J.-P. (1992). Dopaminergic regulation of luteinizing hormone-releasing hormone release at the median eminence level: immunocytochemical and physiological evidence in hens. Neuroendocrinology 55, 290-300. 10.1159/000126128 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

