Fracture healing physiology and the quest for therapies for delayed healing and nonunion
- PMID: 27743449
- PMCID: PMC6120140
- DOI: 10.1002/jor.23460
Fracture healing physiology and the quest for therapies for delayed healing and nonunion
Abstract
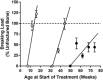
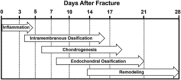
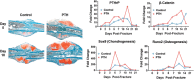
Delayed healing and nonunion of fractures represent enormous burdens to patients and healthcare systems. There are currently no approved pharmacological agents for the treatment of established nonunions, or for the acceleration of fracture healing, and no pharmacological agents are approved for promoting the healing of closed fractures. Yet several pharmacologic agents have the potential to enhance some aspects of fracture healing. In preclinical studies, various agents working across a broad spectrum of molecular pathways can produce larger, denser and stronger fracture calluses. However, untreated control animals in most of these studies also demonstrate robust structural and biomechanical healing, leaving unclear how these interventions might alter the healing of recalcitrant fractures in humans. This review describes the physiology of fracture healing, with a focus on aspects of natural repair that may be pharmacologically augmented to prevent or treat delayed or nonunion fractures (collectively referred to as DNFs). The agents covered in this review include recombinant BMPs, PTH/PTHrP receptor agonists, activators of Wnt/β-catenin signaling, and recombinant FGF-2. Agents from these therapeutic classes have undergone extensive preclinical testing and progressed to clinical fracture healing trials. Each can promote bone formation, which is important for the stability of bridged calluses, and some but not all can also promote cartilage formation, which may be critical for the initial bridging and subsequent stabilization of fractures. Appropriately timed stimulation of chondrogenesis and osteogenesis in the fracture callus may be a more effective approach for preventing or treating DNFs compared with stimulation of osteogenesis alone. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 35:213-223, 2017.
Keywords: chondrogenesis; delayed union; fracture healing; nonunion fracture; osteogenesis.
© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures



References
-
- Mosekilde L, Bak B. 1993. The effects of growth hormone on fracture healing in rats: a histological description. Bone 14:19–27. - PubMed
-
- Tzioupis C, Giannoudis PV. 2007. Prevalence of long‐bone non‐unions. Injury 38:S3–9. - PubMed
-
- Westgeest J, Weber D, Dulai SK, et al. 2016. Factors associated with development of nonunion or delayed healing after an open long bone fracture: a prospective cohort study of 736 subjects. J Orthop Trauma 30:149–155. - PubMed
-
- Marsh D. 1998. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res S22–30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

