Oscillatory Dynamics of Prefrontal Cognitive Control
- PMID: 27743685
- PMCID: PMC5127407
- DOI: 10.1016/j.tics.2016.09.007
Oscillatory Dynamics of Prefrontal Cognitive Control
Abstract
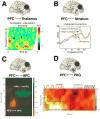
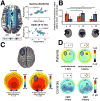
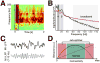
The prefrontal cortex (PFC) provides the structural basis for numerous higher cognitive functions. However, it is still largely unknown which mechanisms provide the functional basis for flexible cognitive control of goal-directed behavior. Here, we review recent findings that suggest that the functional architecture of cognition is profoundly rhythmic and propose that the PFC serves as a conductor to orchestrate task-relevant large-scale networks. We highlight several studies that demonstrated that oscillatory dynamics, such as phase resetting, cross-frequency coupling (CFC), and entrainment, support PFC-dependent recruitment of task-relevant regions into coherent functional networks. Importantly, these findings support the notion that distinct spectral signatures reflect different cortical computations supporting effective multiplexing on different temporal channels along the same anatomical pathways.
Keywords: cross-frequency coupling; large-scale networks; network connectivity; neuronal entrainment; neuronal oscillations; prefrontal cortex.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


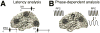
References
-
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. - PubMed
-
- Siegel M, et al. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. - PubMed
-
- Thut G, et al. The functional importance of rhythmic activity in the brain. Curr Biol CB. 2012;22:R658–663. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

