Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels
- PMID: 27743844
- PMCID: PMC5407468
- DOI: 10.1016/j.tibs.2016.09.004
Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels
Abstract
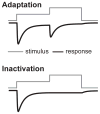
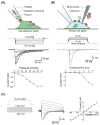
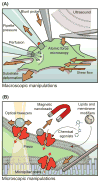
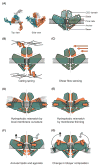
In 2010, two proteins, Piezo1 and Piezo2, were identified as the long-sought molecular carriers of an excitatory mechanically activated current found in many cells. This discovery has opened the floodgates for studying a vast number of mechanotransduction processes. Over the past 6 years, groundbreaking research has identified Piezos as ion channels that sense light touch, proprioception, and vascular blood flow, ruled out roles for Piezos in several other mechanotransduction processes, and revealed the basic structural and functional properties of the channel. Here, we review these findings and discuss the many aspects of Piezo function that remain mysterious, including how Piezos convert a variety of mechanical stimuli into channel activation and subsequent inactivation, and what molecules and mechanisms modulate Piezo function.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

